114 Viral Arthritis
Always take exposure, travel, occupation, and vaccination histories.
Parvovirus B19 is the most common viral arthritis in the United States.
In adults with parvovirus B19 infection, rash may be subtle or absent.
Hepatitis B virus infection presents as an arthritis-urticaria syndrome.
The history of risk behaviors associated with hepatitis C virus infection may be remote.
Viral effects in a given host may depend on host factors such as age, gender, genetic background, infection history, and immune response. The ability of a given virus to infect a host may also depend on the viral mode of host entry, tissue tropism, replication strategy, cytopathologic effects, ability to establish persistent infection, viral expression of host-like antigens, and ability to alter host antigens. Viral modification of the regulation of cellular gene expression may contribute to autoimmunity. Infected cells may die by classic cell necrosis, programmed cell death (apoptosis), or autophagy. Initiation of an immune response to virally encoded antigens on the cell surface may target that cell for destruction and alter cell-cell interactions. The antibody response may generate immune complexes that are deposited locally at the site of viral infection or systemically in synovium. Alternatively, cells may survive, but their behavior may be altered by the expression of viral genes. Transactivation of cellular genes by viral gene products may induce the cell cycle or cytokines that elicit or perpetuate an immune response targeting host cells. Molecular mimicry of host autoantigens by viral proteins may break immune tolerance. Viral components may elicit “danger signals” that trigger an immune response.1,2
Parvovirus B19
Epidemiology
B19 infection is common and occurs worldwide. B19 typically is transmitted by respiratory secretions but may also be transmitted via pooled blood products. Outbreaks commonly occur in late winter and spring, when close contact is most common, although epidemics may also occur in summer and fall. Most B19 infections, especially in children, remain asymptomatic or are diagnosed as nonspecific viral illnesses. Outbreaks tend to occur in 3- to 5-year cycles, representing the time required for a new cohort of susceptible children to enter school. Up to 60% of adults have serologic evidence of past B19 infection.3,4 Susceptible adults in occupations with multiple exposures to children, such as schoolteachers and pediatric nurses, are at greatest risk (up to 50%) of acquiring infection during outbreaks.4,5 Sporadic cases do occur during nonepidemic periods. The diagnosis should be entertained even in the absence of surveillance data suggesting an outbreak.
Pathogenesis
The onset of joint symptoms and rash is associated temporally with the appearance of serum anti-B19 immunoglobulin (Ig)M antibody, suggesting a role for circulating immune complexes during the acute phase of the illness.6 Although little evidence of circulating virus has been noted in patients who have chronic joint symptoms, B19 DNA may be found in the bone marrow and synovium of patients with chronic B19 arthropathy. Persistence in chronic B19 arthropathy may be facilitated by failure to develop IgG antibodies to the N-terminal region of the minor capsid protein VP1, known to encode neutralizing epitopes.6 The presence of antibody to the B19 nonstructural protein NS1 in some cases of chronic B19 arthropathy probably reflects the immune response to NS1 on the surface of B19 virions or NS1 spilled during cell death.7 NS1 protein itself, however, may play a pathogenic role in perpetuating chronic B19 arthropathy through its interaction with cellular genes.8 NS1 protein upregulates in vitro transcription from the interleukin (IL)-6 promoter and from human immunodeficiency virus (HIV) long terminal repeats in the presence of tat and an intact tar element.9,10 A high prevalence of B19 DNA and proteins in synovium from rheumatoid arthritis patients was reported in association with enhanced synovial production of IL-6 and tumor necrosis factor.8 These findings remain controversial.11 B19 may induce apoptosis through NS1, which is known to be toxic to cells.12,13 Production of NS1 in nonpermissive synoviocytes could theoretically induce autoimmunity by disrupting normal patterns of cell interactions and intercellular regulation.
Diagnosis
Clinical Features
The incubation period from B19 infection to symptom onset is 7 to 18 days. B19 causes transient aplastic crisis in the setting of chronic hemolytic anemia.6 In otherwise healthy children, B19 causes erythema infectiosum, or fifth disease, characterized by bright red “slapped cheeks” and a macular or maculopapular eruption on the torso and extremities. Up to 70% of infected children may be asymptomatic; others may have mild flu-like symptoms, including fever, headache, sore throat, cough, anorexia, vomiting, diarrhea, and arthralgia. In adults, the rash tends to be subtler, and the slapped-cheek rash is usually absent. Uncommon dermatologic manifestations include vesicular or hemorrhagic vesiculopustular eruptions, purpura with or without thrombocytopenia, Henoch-Schönlein purpura, and a “socks and gloves” acral erythema. B19 infection may be associated with paresthesias in the fingers and, rarely, with numbness of the toes. Progressive arm weakness has been associated with mild nerve conduction slowing and decreased motor and sensory potential amplitudes. B19 may cross the placenta to infect the fetus, which may develop hydrops fetalis on the basis of B19-induced anemia or viral cardiomyopathy. Less commonly, B19 may cause pancytopenia, isolated anemia, thrombocytopenia, leukopenia, myocarditis, neuropathy, or hepatitis.14 Reports suggest that B19 may be associated with vasculitis, including giant cell arteritis.15,16
Patients with congenital or acquired immunodeficiencies, including prior chemotherapy or acquired immunodeficiency syndrome (AIDS) due to HIV infection, may develop persistent B19 infection with chronic or recurrent anemia, thrombocytopenia, or leukopenia. B19 infection is the leading cause of pure red cell aplasia in patients with AIDS.17,18
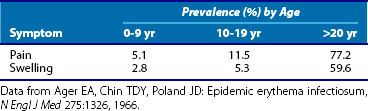
In a study of an erythema infectiosum outbreak in Port Angeles, Washington, in which subjects were identified on the basis of rash, the incidence of arthralgia and joint swelling increased with age (Table 114-1).19 In adults, a severe flu-like illness consisting of fever, chills, malaise, and myalgias may precede or accompany sudden-onset, moderately severe, symmetric polyarthritis in a rheumatoid-like distribution. The arthritis is characterized by prominent involvement of the finger proximal interphalangeal, metacarpophalangeal, wrist, knee, and ankle joints. Within 24 to 48 hours of onset, all affected joints become involved. Axial skeleton involvement is uncommon. Joint symptoms are usually self-limited.
Table 114-1 Prevalence of Joint Symptoms in Fifth Disease by Age: Port Angeles, Washington, 1961-1962
After the initial infection, objective joint swelling, heat, and erythema, when present, tend to resolve over several weeks. A minority of patients have prolonged symptoms that fall into one of two patterns. Approximately two-thirds have continuous morning stiffness and arthralgias with intermittent flares. The remaining patients are symptom free between flares. Chronic B19 arthropathy may last months to years. Pain remains a prominent feature during flares; patients commonly report morning stiffness. Approximately 12% of patients presenting with “early synovitis” have B19 infection; most are women.6
Laboratory Tests
The antibody response is associated with the second phase of clinical illness, characterized by rash and joint symptoms. Onset of the anti-B19 IgG antibody response occurs almost concurrently with the IgM response. The two clinical phases of illness often overlap. Low to moderate titers of rheumatoid factor and anti-DNA, antilymphocyte, antinuclear, and antiphospholipid antibodies may be present initially.20–24
During viremia, immune electron microscopy may detect virions in serum. However, this method is not readily available to clinicians. B19 DNA may be detected during viremia. However, because adult patients usually present after the onset of joint symptoms, the most useful diagnostic test is anti-B19 IgM serology. Radioimmunoassays and enzyme-linked immunosorbent assays have been used to detect B19 antigen and specific antibody to B19 capsid.6,25,26 The anti-B19 IgM antibody response is usually positive for 2 months after the acute illness and may wane shortly thereafter. In some patients, anti-B19 IgM may be detected for 6 months or longer. A positive anti-B19 IgG antibody test in the absence of anti-B19 IgM usually is not diagnostically helpful because of the high seroprevalence of anti-B19 IgG in the adult population. Reports of B19 DNA in normal synovium suggest that testing for B19 DNA in these tissues is of little clinical utility in the absence of anti-B19 IgM.27
Treatment and Outcome
No specific treatment or vaccine has been identified for B19 infection. Therefore, treatment is symptomatic and consists of nonsteroidal anti-inflammatory drugs. Intravenous immunoglobulin has been successful in the treatment of bone marrow suppression and B19 persistence in immunocompromised patients,18 but initial studies suggest that this is not applicable to patients with chronic arthropathy. Long-term prognosis is good. Although subjective arthralgias and morning stiffness may be prolonged, joint destruction is not a feature of chronic B19 arthropathy. The role of B19 as a cofactor in the development of classic erosive rheumatoid arthritis has not been confirmed.
Togaviruses
The family Togaviridae includes the Rubivirus and Alphavirus genera.
Rubella Virus
Rubella virus is the sole member of the genus Rubivirus. It consists of enveloped, single-stranded RNA viruses. The rubella virion is spherical and measures 50 to 70 nm in diameter, with a 30-nm dense core. Envelope glycoproteins form 5- to 6-nm spike-like projections that contain hemagglutination activity.28
Epidemiology
Transmission is by nasopharyngeal secretions, with a peak incidence in late winter and spring. Vaccination has reduced the incidence of rubella outbreaks and has shifted the demographic profile from children to college students and adults. The incubation period from infection to rash is 14 to 21 days. Viremia precedes rash by 6 to 7 days, peaks just before the onset of rash, and clears within 48 hours after the onset of rash. Nasopharyngeal shedding of virus is detectable from 7 days before the appearance of rash until 14 days afterward, but it is maximal from just before the rash until 5 to 6 days later.29
Pathogenesis
Rubella virus can persistently infect synoviocytes and chondrocytes in vitro. An inadequate humoral immune response to specific rubella envelope glycoprotein epitopes may allow rubella virus to persistently infect synovium and lymphocytes in patients with chronic rubella arthritis. The onset of rash and arthritis is concurrent with antibody production, suggesting a role for antibody or immune complexes.29 Concentrations of rubella antibody are higher in synovial fluid than in serum. Synovial lymphocytes from infected individuals spontaneously secrete rubella antibody in vitro, suggesting that an immune response to rubella infection occurs in the joint.30
Diagnosis
Clinical Features.
Joint symptoms commonly occur in women beginning 1 week before or 1 week after the appearance of the rash. Symmetric or migratory arthralgias are more common than synovitis. Morning stiffness is prominent. Joint symptoms usually resolve over a few days to 2 weeks. Proximal interphalangeal, metacarpophalangeal, wrist, elbow, ankle, and knee joints are most frequently affected. Periarthritis, tenosynovitis, and carpal tunnel syndrome may be seen. In some patients, symptoms may persist for months to years.31,32
Live attenuated rubella vaccines have caused a high frequency of postvaccination myalgia, arthralgia, arthritis, and paresthesia—symptoms similar to those seen in natural infection—beginning 2 weeks after inoculation and lasting less than a week. However, in some patients, symptoms may persist for longer than a year. RA27/3, the vaccine strain in current use, may cause postvaccination joint symptoms in 15% or more of recipients.31,32
Two rheumatologic syndromes may complicate natural infection or vaccination in children. In the catcher’s crouch syndrome, a lumbar radiculoneuropathy causes popliteal fossa pain on arising in the morning. Exacerbation of the pain by knee extension encourages the assumption of a baseball catcher’s crouch position. The pain gradually subsides through the day but recurs the next morning. In the arm syndrome, brachial neuropathy causes arm and hand pain and dysesthesias that are worse at night. Both syndromes may occur beginning 1 to 2 months after infection or vaccination, with the initial episode lasting up to 2 months. Episodes recur for up to 1 year but eventually resolve without long-term sequelae.33
Laboratory Tests.
Although rubella may be cultured from tissues and body fluids, including throat swabs, detecting antirubella IgM antibody usually establishes the diagnosis of acute rubella infection. Diagnosis by anti-IgG antibody seroconversion requires paired acute and convalescent sera. IgM and IgG are usually present at the onset of joint symptoms. IgM antibody levels peak 8 to 21 days after symptom onset and wane by 5 weeks. Antirubella IgG rises rapidly over a period of 1 to 3 weeks and is long lived. A single positive IgG serum sample or a set of untitered IgG-positive screens documents only immunity.29
Alphaviruses
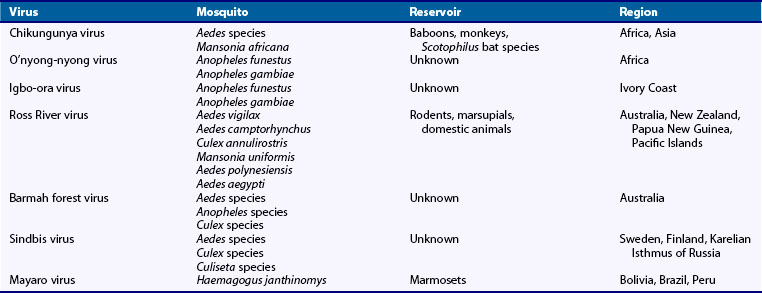
Members of the genus Alphavirus are enveloped, single-stranded RNA viruses transmitted by mosquitoes.35 Several cause acute febrile arthropathy, and their names reflect local appreciation of their clinical impact. For example, chikungunya means “that which twists or bends up” (Tanzania). The related o’nyong-nyong virus means “joint breaker” in the Acholi (Uganda) dialect. Igbo-ora is “the disease that breaks your wings.”
Epidemiology
Chikungunya, o’nyong-nyong, and igbo-ora viruses form a serologically related group. Chikungunya virus was isolated during an epidemic of febrile arthritis in Tanzania between 1952 and 1953. Similar epidemics probably occurred in Africa, Asia, India, Indonesia, and possibly the southern United States as early as 1779.35 Mosquitoes responsible for transmission to humans define its geographic distribution (Table 114-2). A feared consequence of global warming is spread of the geographic range of infected mosquitoes.36–39
Chikungunya fever occurs endemically and in epidemics.40 Outbreaks have been described in the Indian Ocean islands, Malaysia, and Hong Kong.41–44 An outbreak occurred in Italy in 2007. A large-scale outbreak of the serologically related o’nyong-nyong virus occurred in the Acholi province of northwestern Uganda in February 1959; this outbreak spread through Uganda and the surrounding region at a rate of 2 to 3 kilometers daily, affecting more than 2 million people within 2 years.45 After the initial o’nyong-nyong epidemic, clinical disease was not detected again until it re-emerged in the Acholi region in 1996.46 Despite the absence of outbreaks in the intervening years, serologic surveys have demonstrated that o’nyong-nyong virus is endemic.47
Weber’s line is a hypothetical demarcation separating the Australian and Asiatic geographic zones. Antibodies to chikungunya virus are found west of Weber’s line, and Ross River virus antibodies are found only east of it. Ross River virus causes epidemics of fever and rash in Australia, New Zealand, and the western Pacific islands.48 In the Fiji Islands from 1979 to 1980, Ross River virus caused febrile polyarthritis in more than 40,000 individuals.49 In Australia, endemic cases and epidemics occur in tropical and temperate regions annually.50 Most cases occur in Queensland and New South Wales territories, where high rainfall and subsequent increases in mosquito populations usually precede epidemic periods. Infection rates in Australia range from 0.2% to 3.5% per year. Male and female infection rates are similar, but a female predominance has been noted in presenting cases. Most infected adults are symptomatic; the case rate for children is lower. Barmah Forest virus, another alphavirus with an increasing incidence in Australia, may manifest in a fashion similar to Ross River virus.51–56
Individuals involved in outdoor activities or occupations in forested areas in Sweden, Finland, and the neighboring Karelian isthmus of Russia are at greatest risk for infection with Sindbis virus; in those regions, it is known as Okelbo disease, Pogosta disease, and Karelian fever, respectively. Birds are the intermediate host.57 It has also been reported in central Africa, Zimbabwe, South Africa, and Australia in sporadic cases or small outbreaks.35
Mayaro virus, first recognized in Trinidad in 1954, is endemic in the tropical rain forests of Bolivia, Brazil, and Peru. Cases have been imported into the United States in individuals traveling from endemic areas.58
Diagnosis
Clinical Features.
Chikungunya fever presents with an explosive onset of high fever and severe arthralgia after a 1- to 12-day incubation period. The fever lasts 1 to 7 days. Typically, a macular or maculopapular, sometimes pruritic, rash on the torso, extremities, and occasionally the face, palms, and soles occurs on day 2 to 5 of illness as the patient defervesces. The rash may last 1 to 5 days and may recur with fever. Isolated petechiae and mucosal bleeding may occur. In some patients, involved skin desquamates.59,60 Chemosis is prominent. Headache, photophobia, retro-orbital pain, pharyngitis, anorexia, nausea, vomiting, and abdominal pain may be present. Diffuse myalgia and back and shoulder pain are common. Migratory polyarthralgia, stiffness, and swelling affect predominantly the small joints of the hands, wrists, feet, and ankles. Large joints are less severely affected. Previously injured joints may be disproportionately affected. Large effusions are uncommon. Symptoms in children tend to be milder. Low-titer rheumatoid factor may be found in those with long-standing symptoms.
O’nyong-nyong fever is clinically similar to chikungunya fever.61,62 In 1984, igbo-ora caused an epidemic of fever, myalgias, arthralgias, and rash in four Ivory Coast villages. Sequencing of isolates from the 1996 outbreak of o’nyong-nyong fever suggested that igbo-ora virus is a variant of o’nyong-nyong virus.46
Ross River virus polyarthralgia is severe, incapacitating, and often migratory and asymmetric.63 Symptoms follow a 7- to 11-day incubation period. Finger interphalangeal and metacarpophalangeal joints, wrists, knees, ankles, shoulders, elbows, and toes are often involved. Polyarticular swelling and tenosynovitis are common. Arthralgias are worse in the morning and after inactivity. Rash is macular, papular, or maculopapular and may be pruritic. Vesicles, papules, or petechiae are typically seen on the trunk and extremities. The palms, soles, and face may be involved. Rash typically appears 1 to 2 days before joint symptoms, but it may occur anywhere from 11 days before to 15 days after the onset of arthralgias, and it resolves by fading to a brownish discoloration or by desquamation. Half of patients have no fever, and those who do may have only modest fever lasting 1 to 3 days. Nausea, headache, and myalgia are common. Respiratory symptoms, mild photophobia, and lymphadenopathy may occur. Up to a third of patients have paresthesias and palm or sole pain. Carpal tunnel syndrome may be seen. Arthritis is less common and less prominent in Barmah Forest virus infection than in Ross River virus infection, but the rash is more common and florid.64,65
Rash and arthralgia are the presenting symptoms in Sindbis virus infection, although one may precede the other by a few days. Constitutional symptoms are usually mild and include low-grade fever, headache, fatigue, malaise, nausea, vomiting, pharyngitis, and paresthesias. A macular rash typically begins on the torso and then spreads to arms and legs, palms, soles, and occasionally the head. Macules evolve to form papules that tend to vesiculate. Vesiculation is prominent on pressure points, including the palms and soles. As the rash fades, a brownish discoloration is left. Vesicles on the palms and soles may become hemorrhagic. The rash may recur during convalescence.66
A Mayaro virus outbreak in Belterra, Brazil, in 1988 was characterized by sudden onset of fever, headache, dizziness, chills, and arthralgias in the wrists, fingers, ankles, and toes. The clinical attack rate was 80%. Joint swelling, unilateral inguinal lymphadenopathy, and leukopenia may be present. A maculopapular rash on the trunk and extremities lasts about 3 days.67
Laboratory Tests.
The diagnosis of alphavirus infection requires laboratory confirmation. Any febrile patient residing in or returning from an endemic area should undergo a laboratory investigation. Chikungunya virus may be isolated from serum on days 2 through 4 of illness.68 Neutralizing antibody, hemagglutination inhibition activity, and complement fixation tests may be used to detect antibodies. Chikungunya virus–specific IgM antibodies may be found for 6 months or longer.69 O’nyong-nyong virus may be isolated by intracerebral injection into suckling mice, in which it produces alopecia, rash, and runting. Hemagglutination inhibition or complement fixation tests identify o’nyong-nyong virus.70,71 Because chikungunya and o’nyong-nyong viruses are closely related serologically, mouse antisera raised to chikungunya virus or o’nyong-nyong virus react equally well with o’nyong-nyong virus, but o’nyong-nyong antisera do not react well with chikungunya virus. Molecular detection methods have improved diagnostic specificity.72–75 Specific reverse transcriptase polymerase chain reaction–based assays have been developed for viral RNA detection.73,76
In chikungunya fever, synovial fluid shows decreased viscosity, poor mucin clot, and 2000 to 5000 white blood cells/mm3. Ross River virus has been isolated only from antibody-negative sera. In Australian epidemics before 1979, patients were antibody positive at the time of presentation. In contrast, patients during the Pacific island epidemics of 1979 to 1980 remained viremic and seronegative for up to 1 week after the onset of symptoms. Synovial fluid cell counts range from 1500 to 13,800 cells/mm3, predominantly monocytes and vacuolated macrophages.77 Barmah Forest virus infection is confirmed by rising titers of specific IgG.64 Diagnosis of Sindbis virus infection is confirmed by specific serology.
Pathogenesis
Little is known about the pathogenesis of chikungunya fever or arthritis. Involved skin shows erythrocyte extravasation from superficial capillaries and perivascular cuffing. The virus adsorbs to human platelets, causing aggregation, suggesting a mechanism for bleeding. Synovitis probably results from direct viral infection of synovium. In one patient with chronic arthropathy, the synovium appeared atrophic on arthroscopy and was histologically normal.78 The mechanisms of o’nyong-nyong virus pathogenesis are unknown. However, the virus was isolated from peripheral blood mononuclear cells in a patient in Chad.79
Ross River virus antigen may be detected early in monocytes and macrophages by immunofluorescence, but intact virus is not identifiable by electron microscopy or cell culture.80 Erythematous and purpuric rashes show mild dermal perivascular mononuclear cell infiltrates, mostly T lymphocytes. Purpuric areas also show erythrocyte extravasation. Viral antigen may be detected in epithelial cells in erythematous and purpuric skin lesions and in perivascular zones in erythematous lesions.81
Sindbis virus has been isolated from a skin vesicle in the absence of viremia. Skin lesions show perivascular edema, hemorrhage, lymphocytic infiltrates, and areas of necrosis. Anti–Sindbis virus IgM may persist for years, raising the possibility that Sindbis virus arthritis is associated with viral persistence.82
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree