55 Antinuclear Antibodies
Antinuclear antibodies (ANAs) include an ever-growing diversity of autoantibodies directed against multiple intracellular antigens, classically consisting of nuclear specificities such as deoxyribonucleic acid (DNA) or small nuclear ribonucleoproteins (snRNPs).1,2 ANA diseases (Table 55-1) include syndromes whose patients are characterized by an unusually high prevalence of ANAs, often screened for by the fluorescent antinuclear antibody (FANA) test: systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and mixed connective tissue disease (MCTD). The prevalence of ANAs in polymyositis (PM), dermatomyositis (DM), and Sjögren’s syndrome (SS) has been reported to be somewhat lower than in other ANA diseases, but they are often grouped together because they share similar target antigens and therefore presumably similar causes. For decades, ANA testing has remained an important diagnostic and prognostic tool for these connective tissue diseases and has provided routine assays in the evaluation of patients with suspected rheumatic disease. However, these autoantibodies arise in a variety of infectious, inflammatory, and neoplastic diseases, as well as in normal individuals; therefore some knowledge regarding their intricacies and limitations of the assays is required for appropriate clinical application. This chapter summarizes the more common ANA specificities, outlining their history, implicated relevance to pathogenesis, methods of detection, and clinical disease associations, as well as the biology of their target autoantigens, in an effort to help guide the clinical utility of testing for these specificities.
Table 55-1 Antinuclear Antibody (ANA)-Associated Diseases and Related Conditions
| Condition | Patients with ANAs (%) |
|---|---|
| Diseases for Which ANA Testing Is Helpful for Diagnosis | |
| Systemic lupus erythematosus | 99-100 |
| Systemic sclerosis | 97 |
| Polymyositis/Dermatomyositis | 40-80 |
| Sjögren’s syndrome | 48-96 |
| Diseases in Which ANA Is Required for Diagnosis | |
| Drug-induced lupus | 100 |
| Mixed connective tissue disease | 100 |
| Autoimmune hepatitis | 100 |
| Diseases in Which ANA May Be Useful for Prognosis | |
| Juvenile idiopathic arthritis | 20-50 |
| Antiphospholipid antibody syndrome | 40-50 |
| Raynaud’s phenomenon | 20-60 |
| Some Diseases for Which ANA Typically Is Not Useful | |
| Discoid lupus erythematosus | 5-25 |
| Fibromyalgia | 15-25 |
| Rheumatoid arthritis | 30-50 |
| Relatives of patients with autoimmune disease | 5-25 |
| Multiple sclerosis | 25 |
| Idiopathic thrombocytopenic purpura | 10-30 |
| Thyroid disease | 30-50 |
| Patients with silicone breast implants | 15-25 |
| Infectious disease | Varies widely |
| Malignancies | Varies widely |
| Healthy (“Normal”) Individuals | |
| ≥1 : 40 | 20-30 |
| ≥1 : 80 | 10-12 |
| ≥1 : 160 | 5 |
| ≥1 : 320 | 3 |
Adapted from Kavanaugh A, Tomar R, Reveille J, et al, American College of Pathologists: Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens, Arch Pathol Lab Med 124:71–81, 2000.
History
Key Point
In the first formal description of an ANA-related phenomenon in 1948, bone marrow specimens from a patient with SLE were found to contain lupus erythematosus (“LE”) cells, which were subsequently found useful in the diagnosis of SLE and drug-induced lupus, as well as SS and rheumatoid arthritis (RA).3 LE cells were soon discovered to be the result of a plasma factor, autoantibody against deoxyribonucleoprotein, which opsonized and/or induced apoptosis in free-cell nuclei, resulting in antibody sensitization and phagocytosis by polymorphonuclear neutrophils. In 1957, indirect immunofluorescence allowed the development of the FANA as a more sensitive assay for SLE and related diseases.4 Finer distinction of autoantibody reactivities detected by FANA testing led to the description of Smith (Sm), nuclear ribonucleoprotein (nRNP), Ro/Sjögren’s syndrome (SSA), and La/SSB specificities, which later gained further biologic prominence with the demonstration that their autoantigens play prominent roles in cellular homeostasis (e.g., snRNPs, targets of anti-Sm and anti-nRNP) and in regulation of premessenger RNA splicing.5 Subsequent investigations have revealed an ever growing array of autoantigens (Table 55-2), many of which remain largely uncharacterized. Thus, ANAs not only serve as diagnostic markers in autoimmunity but to this day have greatly aided studies on cellular biochemistry.
Relevance of ANTINUCLEAR ANTIBODIES to Disease Pathogenesis
Key Point
Because of the characteristic presence of these autoantibodies among the ANA diseases, ANAs have long been speculated to play a role in disease pathogenesis. Anti-DNA antibodies, for instance, have been suspected to promote inflammation in SLE nephritis via immune complex deposition, direct binding to cross-reactive glomerular antigens, and/or intracellular penetration and induction of cellular toxicity.6 Similarly, ribonucleoprotein antibodies such as anti-Ro/SSA, anti-La/SSB, and anti-Sm have been implicated in the pathogenesis of cutaneous or cardiac manifestations by penetrating live cells and/or binding to exposed antigens in the skin and/or the heart.7,8 Sera containing anti-Scl-70 (topoisomerase I) activity can induce high levels of interferon (IFN)-α, correlating with diffuse cutaneous scleroderma and lung fibrosis9; also, anti-Jo-1- or anti-Ro/SSA-positive sera from myositis patients have been demonstrated to induce type I IFN and/or intercellular adhesion molecule (ICAM)-1 on endothelial cells.10,11
However, autoantibodies alone appear insufficient to account for disease pathogenesis. Induction of type I IFN activity by anti-Ro/SSA–containing sera, for instance, appears restricted to patients with SLE or SS, not asymptomatic individuals,12 and surface binding of topoisomerase I may be required for a pathogenic effect of anti-Scl-70 antibodies.13 This may reflect additional biologic issues among or effects of the autoantigens themselves, such as novel conformations or epitopes: for instance, a proteolytically sensitive conformation of histidyl-transfer RNA synthetase (HisRS), the target of the pulmonary fibrosis–related Jo-1–specific antibody, has been described in the lung,14 and an apoptope (epitope expressed on apoptotic cells) of Ro/SSA may be specific to SLE, suggesting a unique role of apoptosis in disease pathogenesis.15 The autoantigens themselves may have unique biologic functions: 60-kD Ro/SSA, for instance, may serve as a receptor for the antiphospholipid-related β2-glycoprotein I, and this dynamic may account for differences in Ro antibody pathogenicity.16 However, many autoantigens likely have intrinsic proinflammatory properties, such as stimulation of innate inflammation by DNA and RNA via Toll-like receptors (TLRs) 3, 7, and 9,17,18 or induction of smooth muscle responses by the centromere protein CENP-B via CCR3.19 Apparent remission of SLE in a patient has been correlated with loss of TLR responsiveness, antibody deficiency, and disappearance of anti-DNA, supporting such concepts.20 Thus the pathogenesis of the connective tissue diseases appears to reflect a complex interplay between direct inflammatory or other biologic effects of the autoantigens and consequences of autoantibody responses.
Methods of Detection
Immunofluorescence
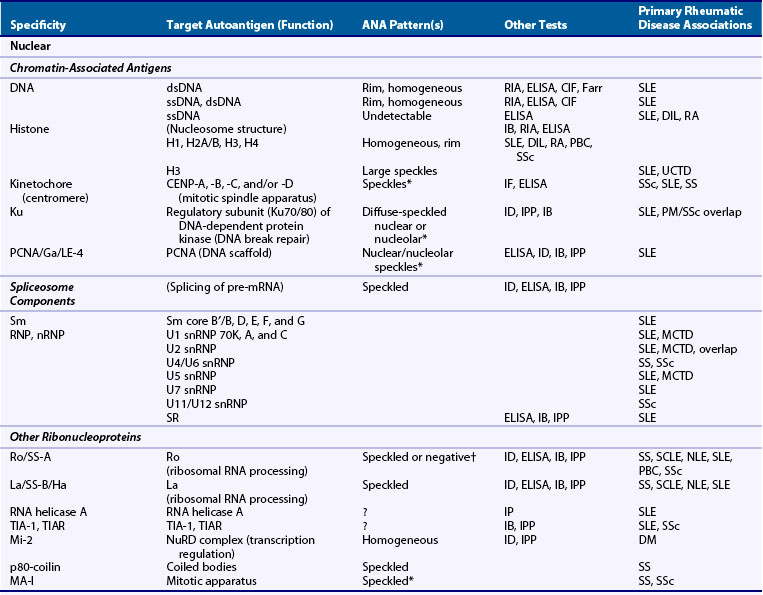
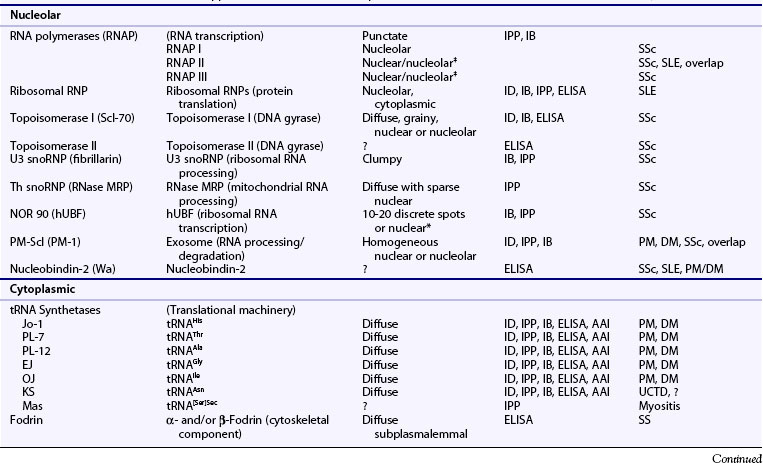
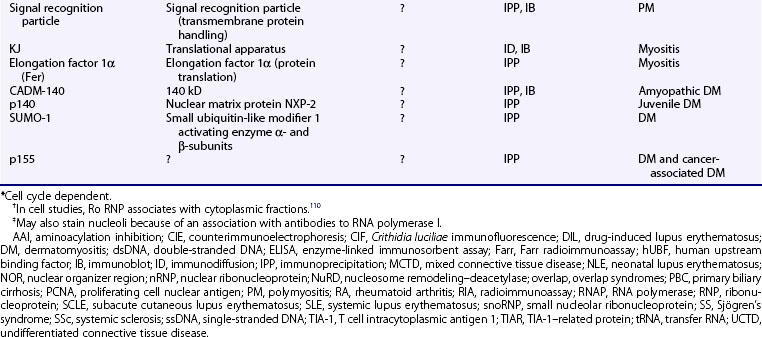
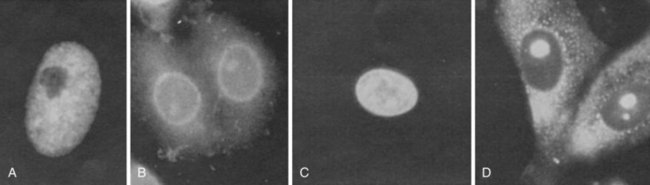
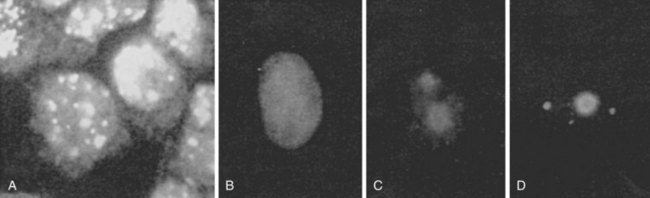
The FANA provides a rapid yet highly sensitive screening method for ANA detection and remains the gold standard for clinical testing.21,22 Here, test sera at varying dilutions (typically serially increasing by twofold) are incubated with substrate cells, and bound antibodies are detected by fluorescein-conjugated anti–human immunoglobulin G (IgG), followed by visualization via a fluorescence microscope. Results typically are reported by two parameters—pattern and titer—with any pattern of reactivity at a titer of 1 : 40 or greater considered positive.23 The former includes one or more morphologic descriptors that typically reflect localization of the respective autoantigen(s) (see Table 55-2; Figures 55-1 and 55-2). Titer is generally reported as the last dilution at which an ANA pattern is detectable, but such an assessment has been considered somewhat imprecise and subjective, and interlaboratory standardization has not been widely instituted: Attempts to standardize the protocol have included computer-based fluorescent image quantification, subjective optical scales, and the use of standardized sera to define international units (IU/mL), although this varies by laboratory.
FANA results must always be interpreted in light of the particular substrate used by individual clinical laboratories. Many laboratories continue to use heterogeneous substrates such as rodent liver or kidney tissues; although such sections possess the advantage of eliminating interference from blood-group antibodies, heterophile antibodies, or passenger viruses, cultured cell lines such as HEp-2 cells have remained a gold standard substrate owing to their higher concentrations of nuclear and cytoplasmic antigens and standardization of use.21–24 To minimize the influence of other variables, such as quality of reagents, including fluorescein-conjugated anti–human IgG, as well as the microscope, several laboratory practices have been recommended23,25: (1) performance of the test on serum stored at 4° C for up to 72 hours or at −20° C or below indefinitely; (2) use of acetone-fixed HEp-2 cells as substrate, because ethanol and methanol fixation may remove Ro/SS-A, and mouse or rat tissues contain little Ro/SS-A and do not reveal antibodies to several organelles characteristic of proliferating cells, such as centromeres (kinetochores); (3) use of IgG-specific anti-Ig fluorescein isothiocyanate (FITC) conjugates with an FITC-to-protein ratio of approximately 3.0, an antibody-to-protein ratio of 0.1 or greater, specific antibody content of 30 to 60 µg/mL, and working dilution determined by titration of reference sera with known patterns and end point titers; and (4) use of reference sera, as provided by the World Health Organization or the Centers for Disease Control and Prevention.
Enzyme-Linked Immunosorbent Assay
ELISAs provide highly sensitive and rapid techniques for the detection of autoantibodies: They are commonly used for the detection of specific ANAs, such as anti-DNA and “extractable nuclear antigen” (ENA) autoantibodies (anti-Sm, anti-Ro/SSA, anti-La/SSB, and anti-RNP), often in a “reflex” manner upon detection of a positive screening FANA test. With this technique, test sera are incubated in wells precoated with purified target antigen; bound antibodies are detected via an enzyme-conjugated anti–human immunoglobulin antibody, followed by color visualization with the appropriate enzyme substrate. The popularity of this technique has resulted from the commercial availability of ELISA kits and the ability to perform these assays on a multiplex platform, enabling large numbers of clinical specimens to be processed quickly at reasonably low cost. As a result, many laboratories also use such solid-phase immunoassays instead of FANA for the screening ANA test; however, such practice is limited by the number of displayed autoantigens (typically 8 to 10), resulting in reduced sensitivity compared with FANA.21,22 Conversely, because the ELISA technique can denature autoantigens, ELISAs can produce some false-positive results, and confirmation may require further testing. Therefore recognition of the local technique used for detection of ANAs is critically important for their clinical application in diagnosis and/or prognosis.
Anti-DNA Antibody Tests
Anti-DNA antibodies warrant special consideration owing to their wide range of autoantigenic epitopes and their assay difficulties.6 Antibodies that recognize denatured single-stranded (ss)DNA, which are less specific for rheumatic disease, bind free purine and pyrimidine base sequences; SLE-specific antibodies that recognize native, double-stranded (ds)DNA bind the deoxyribose phosphate backbone or the rarer, conformation-dependent left-handed helical Z-form. Two methods to ensure the use of native dsDNA in anti-DNA tests include digestion with S1 nuclease, which removes overhanging ssDNA ends, and chromatography on a hydroxyapatite column, which separates single-stranded segments from dsDNA. Unfortunately, despite such efforts, native DNA may spontaneously denature, especially when bound to plastic ELISA plates; this may account for the results of several reports that revealed a relative lack of specificity of anti-dsDNA antibodies for SLE. Therefore reliable assays must ensure the integrity of dsDNA.
Other Assays
Several additional assays for the determination of ANA specificity have been employed in clinical and/or basic science studies.26 Such techniques include immunodiffusion and counterimmunoelectrophoresis techniques, two relatively insensitive assays used in many clinical studies associating ANA specificities (especially ENAs) with disease manifestations and outcome; immunoprecipitation and immunoblot, two sensitive and specific assays predominantly confined to research settings; and enzyme inhibition assays (e.g., inhibition of topoisomerase I by anti-Scl-70, inhibition of RNA splicing by anti-snRNP), which include highly specialized techniques to characterize ANAs functionally. Such assays have not achieved widespread use in diagnostic laboratories because of their cumbersome and/or highly specialized natures.
Interpretation of the FLUORESCENT ANTINUCLEAR ANTIBODY (FANA) TEST
Pattern
Patterns of staining by FANA are often reported as homogeneous, speckled, or rim/peripheral when nuclear staining is present, but they may also be reported as cytoplasmic, centromere, or nucleolar, often reflecting intracellular localization of the target antigen(s) (see Table 55-2 and Figures 55-1 55-2 and 55-3). The presence of unusual patterns may be particularly helpful in appropriate clinical settings, such as the presence of a centromere pattern in a patient with features of systemic sclerosis, suggesting anticentromere antibodies, or a cytoplasmic pattern in a patient with features of myositis, suggesting anti–transfer RNA (tRNA) synthetase antibodies. However, the presence or absence of patterns is not always highly accurate in predicting specificity,27 and non-nuclear patterns may not be reported at all by some laboratories, including rare patterns such as nuclear dot, Golgi, or antimitochondrial antibodies.1,28 Furthermore, the role of the FANA pattern in predicting target autoantigen specificities has been largely supplanted by widely clinically available autoantigen-specific ELISAs. As a result, the presence of any such patterns serves as evidence in an appropriate clinical setting of non–organ-specific autoimmunity, which may warrant further evaluation; however, speculation regarding the significance of a specific pattern may be worthwhile in only a select number of cases.
Titer
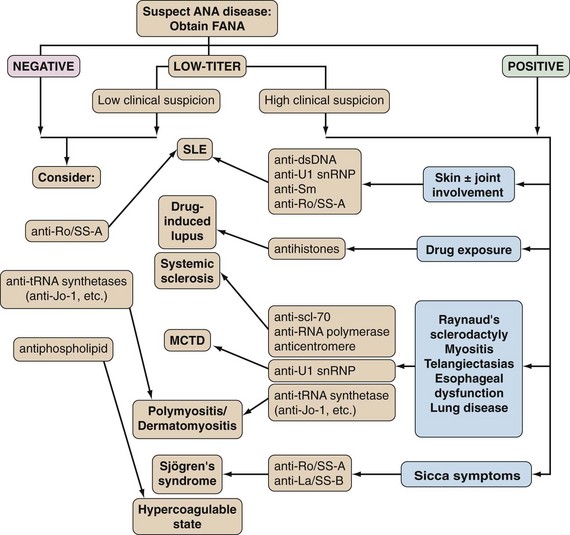
Although the widely accepted cutoff for FANA positivity has remained 1 : 40, greater clinical significance generally has been thought to correlate with higher titers.23 Normal individuals, usually older and female persons, and relatives of persons with connective tissue diseases produce positive FANAs at a frequency sometimes exceeding 30% (see Table 55-1).29,30 Although these patients often possess titers of less than 1 : 320 with homogeneous staining patterns, many subjects possess higher titers yet remain clinically asymptomatic for years,31 and occasional SLE patients may demonstrate negative FANAs; this is perhaps a more frequent observation if they possess isolated anti-Ro or anti-ssDNA antibodies and/or if the laboratory uses rat or mouse tissues.32,33 As a result, high- versus low-titer FANA results may not be of sufficient clinical significance to warrant differential subsequent evaluation. Rather, a positive screening FANA, of any titer, requires clinical correlation.
Diseases Associated with Antinuclear Antibodies
Systemic Lupus Erythematosus
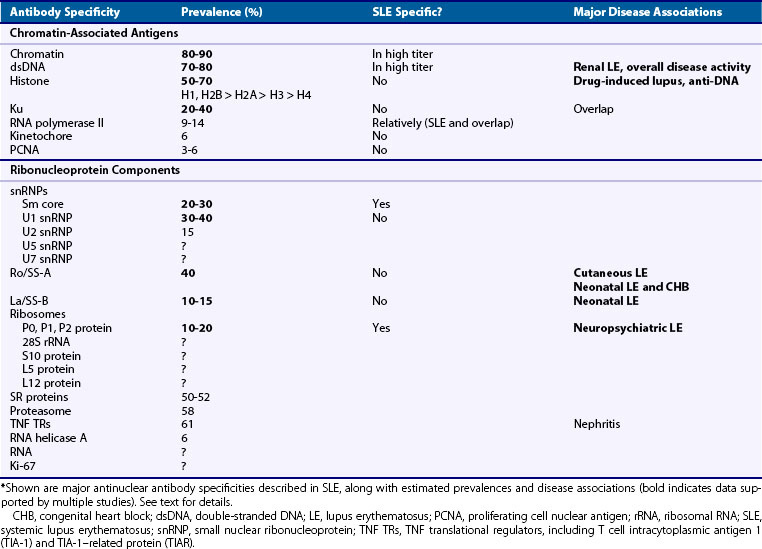
ANAs remain a hallmark of SLE: although past studies have reported FANA frequencies as low as 90%, the test is positive in more than 99% of patients with the use of current methods.34 SLE often evokes autoantibodies against a seemingly endless range of antigens in many cellular locations, but most SLE autoantigens reside in the nucleus and may be broadly categorized into chromatin-associated versus ribonucleoprotein antigens (Table 55-3; also see Table 55-2), which may facilitate disease subclassification and prognosis.35
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree