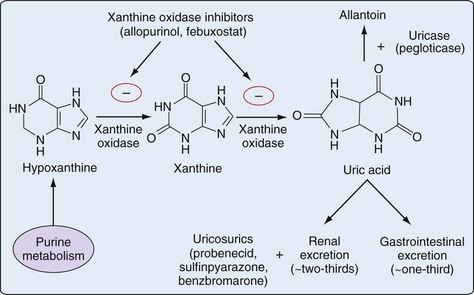
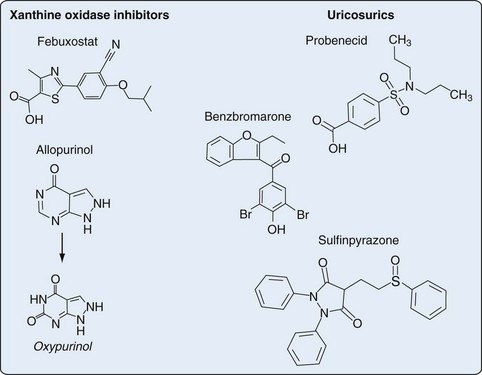
65 Antihyperuricemic Agents
Gout is among the most common forms of inflammatory arthritis, affecting between 1% and 3% of most populations with prevalence rates as high as 6% to 7% in older men.1 The health burden posed by gout is likely to grow with reports suggesting up to a twofold increase in prevalence in recent decades alone.2,3 The rapid growth in gout incidence appears to be largely attributable to a near endemic increase in hyperuricemia. Hyperuricemia is the serum concentration above which uric acid (UA) precipitates into monosodium urate crystals typically defined as serum levels of 6.8 mg/dL or 405 µmol/L or greater, resulting from renal “underexcretion” in most cases (≈80%) with the remainder due to “overproduction.” UA is the end-product of purine degradation, and circulating concentrations reflect the complex interactions of dietary purine intake, endogenous production, and elimination.
Treatment of chronic gout is based primarily on the use of urate-lowering therapy (ULT). Available ULTs include (1) xanthine oxidase (XO) inhibitors (allopurinol, febuxostat), (2) uricosurics (probenecid, benzbromarone, sulfinpyrazone), and (3) uricases (pegloticase) (Table 65-1). This chapter focuses primarily on the most common rheumatic indication for ULT—treatment of hyperuricemia in gout. Optimal use of ULT in gout, regardless of the specific agent used, requires careful consideration of several factors, as detailed in the following sections.
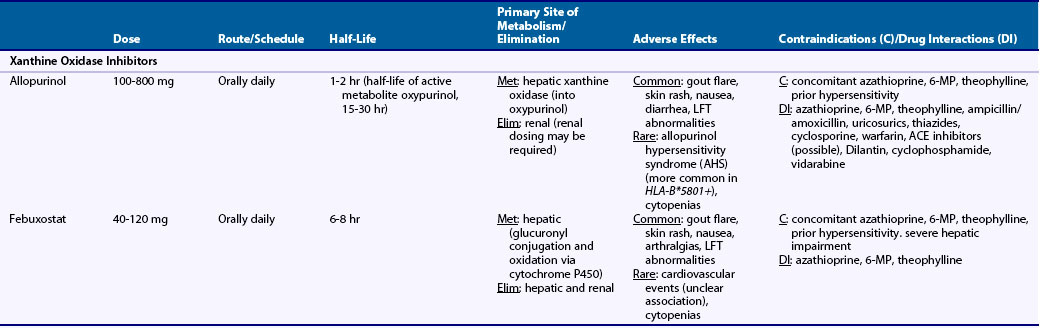
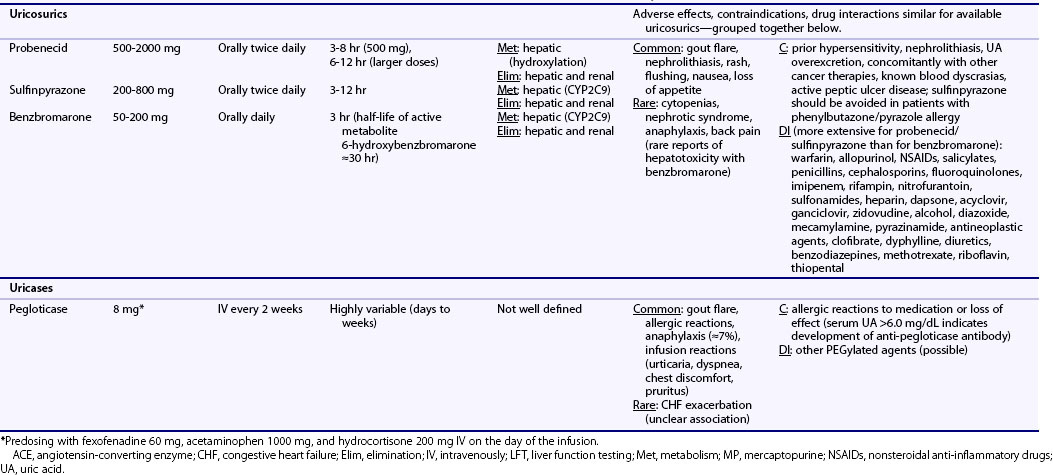
Table 65-1 Dosing and Safety Information for Currently Available Urate-Lowering Therapies in the Management of Gout
Nonpharmacologic Treatment of Hyperuricemia
The importance of education and lifestyle advice pertaining to weight loss, select dietary restrictions, and reduced alcohol intake has been emphasized in recent gout management guidelines.4 Despite a growing list of dietary factors implicated in hyperuricemia and gout, investigations of the effects of dietary interventions on health outcomes in gout are lacking. Furthermore, evidence suggests that such interventions in isolation yield only modest results and suffer from lack of widespread patient acceptance.5 In addition to dietary modifications that include reducing the intake of dietary purines, fructose, beer, and liquor, weight loss represents an important goal for overweight gout patients. Although weight loss may lead to reductions in serum UA,6 its impact on serum UA appears to be modest and may be insufficient alone in many gout patients.
Patient Selection and Timing of Treatment Initiation
Consensus indicates that hyperuricemia should be treated in gout patients with recurrent and frequent flares, tophi, and/or radiographic changes consistent with gout.4,7 For patients with acute gout, ULT should be initiated only after the acute inflammation has resolved because ULT initiation during a flare can amplify the duration and magnitude of symptoms. Conversely, the development of a gout flare complicating ongoing ULT is not an indication to discontinue or hold ULT. Results from a cost-effectiveness analysis in nontophaceous gout, involving a hypothetical patient cohort, suggest that the institution of allopurinol is cost-effective in patients presenting with two or more acute gout flares within a 1-year period.8 Available ULTs are not approved for the treatment of asymptomatic hyperuricemia in the absence of gout.
Duration of Urate-Lowering Therapy
In asymptomatic gout patients successfully treated with prior ULT, withdrawal of therapy often results in an abrupt increase in serum UA, and recurrent attacks occur in approximately one-third of patients within 2 years.9 Similarly, reductions from continuous ULT to an “intermittent” regimen in previously stable gout patients lead to significantly higher flare rates,10 and ULT discontinuation in the setting of tophaceous gout leads to recurrent gout flares in a vast majority and to recurrent tophi in nearly half of patients.11 Taken together, these reports suggest that ULT administration should be unabated and “lifelong” in a majority of gout patients.
Target Serum Urate Goals
Evidence suggests that lowering and sustaining serum UA below 6.0 mg/dL (<360 µmol/L), a treatment goal advocated in recent gout treatment guidelines,4 leads to improved long-term outcomes in gout. ULT reduces the long-term risk of recurrent flare by approximately 60% for each 1 mg/dL decrease in serum urate.12 Additional evidence suggests that reaching and maintaining serum UA concentrations below 6.0 mg/dL is important for depletion of total body urate stores,13 with recognition that lower treatment thresholds (<5.0 mg/dL or <300 µmol/L) have been advocated by some.14 It is important to recognize that these target goals often fall well below the upper limit of normal for UA in clinical laboratories that define ranges based on population-based distributions.
Anti-inflammatory Prophylaxis with Urate-Lowering Therapy
Rebound gout is the most common adverse effect with ULT regardless of the agent used, rendering anti-inflammatory prophylaxis a key component of successful gout treatment. Gout flares complicating ULT are thought to be due to reduced serum UA concentrations that result in mobilization of urate from tissue deposits. In fact, the frequency of treatment-related gout flares appears to be greater with more rapid and potent urate-lowering interventions.15 Both colchicine and naproxen are effective in reducing gout flares during ULT initiation (this is covered in greater detail in Chapter 95).16–18 Results from Borstad and associates17 suggest that anti-inflammatory prophylaxis with low-dose oral colchicine (0.6 mg twice daily) protects against rebound gout flares and may need to be continued for at least 6 months following ULT initiation. Although frequently used in gout flare prevention, the efficacy of other anti-inflammatory agents in this setting, including low-dose glucocorticoids and alternative nonsteroidal anti-inflammatory drugs (NSAIDs), has not been defined. Under active investigation for the treatment of acute gout, interleukin-1 inhibition may also represent an alternative means of prophylaxis with ULT initiation.
Xanthine Oxidase Inhibition
Allopurinol
Available for more than 40 years, allopurinol accounts for a vast majority of ULT prescriptions.1 In addition to its established track record in gout care, allopurinol offers several potential advantages: (1) relatively low cost, (2) once-daily oral administration in most cases, (3) effectiveness in patients who “underexcrete” and in those who “overproduce” UA, (4) a favorable safety profile, and (5) potential effectiveness in patients with renal impairment. Allopurinol treatment results in significant declines in serum UA,18–20 decreased gout flare rates,18–26 and declines in tophus area.19
Role in Rheumatic Disease and Indications
Approved indications for allopurinol include (1) treatment of hyperuricemia in gout, (2) management of malignancy (most often leukemia or lymphoma) in patients undergoing cancer treatment that results in marked increases in serum/urinary UA (allopurinol is stopped once UA overproduction is absent), and (3) management of calcium oxalate–associated nephrolithiasis with daily urinary UA excretion greater than 800 mg/day in men and greater than 700 mg/day in women. Although not approved for the treatment of asymptomatic hyperuricemia in the absence of gout, evidence suggests that allopurinol use may lead to other health benefits. Hyperuricemia is independently associated with cardiovascular morbidity and mortality,27–30 giving rise to speculation that ULT could be cardioprotective.31 In a placebo-controlled study of pediatric essential hypertension, allopurinol use resulted in significant, albeit modest, declines in blood pressure.32 XO inhibition via allopurinol also has been shown to improve endothelial function, improving measures of local and systemic blood flow,33 and has been associated with improvement in renal function in at-risk populations.34,35
Chemical Structure and Mechanism of Action
An antimetabolite in simple organisms, allopurinol inhibits XO, a key enzyme in purine catabolism, but does not inhibit the biosynthesis of purines in humans (Figures 65-1 and 65-2).
Pharmacology
Pharmacologic characteristics of the ULTs are summarized in Table 65-1. Allopurinol is approximately 90% absorbed from the gastrointestinal (GI) tract and is metabolized into oxypurinol, its active metabolite. Peak serum concentrations for allopurinol and oxypurinol are achieved within approximately 1 to 2 hours and 4 to 5 hours, respectively. The plasma half-life of allopurinol is relatively brief (1 to 2 hours), although the half-life of oxypurinol is substantially longer (≈15 hours or longer), allowing for once-daily dosing. Allopurinol is eliminated primarily via glomerular filtration, while oxypurinol undergoes some degree of renal tubular reabsorption. With renal mechanisms primarily responsible for drug elimination, the plasma half-life of allopurinol and, to a greater degree, oxypurinol is increased with renal injury.
Dose and Drug Administration
Allopurinol is available as 100-mg and 300-mg pills for once-daily oral administration (see Table 65-1), with recognition that split dosing has been advocated for daily doses of 600 mg or greater. Used also in the treatment or prevention of tumor lysis syndrome, allopurinol is available for intravenous administration. Approved at daily doses as high as 800 mg, allopurinol is rarely given at doses exceeding 300 mg/day.36 It is well established that only a modest proportion of patients achieve a target serum UA less than 6.0 mg/dL with allopurinol 300 mg/day. Using a target serum UA threshold of less than 5.0 mg/dL, investigators showed that only one-fourth of gout patients achieve this goal with 300 mg of daily allopurinol—a proportion that increased to 78% with a daily dose of 600 mg.37 The limitation of “standard” dose allopurinol has been borne out in recent randomized clinical trials that have compared fixed daily doses of 300 mg to febuxostat in various doses. In those studies, approximately 40% of allopurinol-treated gout patients achieved a final study urate level of less than 6.0 mg/dL.18,19 Recent gout treatment guidelines recommend that allopurinol should be started at low doses (e.g., 100 mg daily) and increased by 100 mg every 2 to 4 weeks as required to achieve a target urate concentration.4 Combined data from two studies suggest that each 100-mg increment in allopurinol is associated with an additional decline in serum urate of approximately 1.0 mg/dL.38,39 Although no data directly support the “start low and go slow” approach over the “fixed-dose” approach, it has been suggested that the former strategy could reduce the incidence of rebound gout flares and mitigate treatment-related toxicity.4
General consensus indicates that initial allopurinol dosing should be adjusted for diminished renal function,4,7,14 which prolongs the plasma half-life of oxypurinol. With age-related increases in gout incidence, the issue of renal dosing has added relevance for the elderly with age-associated declines in kidney function. Commonly cited dosing algorithms suggest administering an initial daily dose of 100 mg or less for patients with a glomerular filtration rate (GFR) below 20 mL/min, and even lower doses for those with more severe renal impairment.40 Whether existing dosing guidelines preclude the use of incremental dosing beyond these recommended renal thresholds is controversial. Renal dosing guidelines that have been promulgated40 are not evidence based and are founded largely on a single retrospective case series showing that patients who developed allopurinol hypersensitivity syndrome (AHS) were more likely to have renal insufficiency. Indeed, many patients with chronic kidney disease (CKD) have developed AHS even with “appropriately” dosed allopurinol.41,42 In their review of 120 gout patients receiving allopurinol, more than half (57%) required daily doses above the “renal threshold” recommended by Hande and co-workers40—a strategy reported to be well tolerated in a majority of patients.43
Toxicity
AHS, an uncommon but potentially fatal treatment complication, is a febrile illness characterized by the presence of an erythematous desquamating rash (similar to Stevens-Johnson), eosinophilia, and end-organ damage, including hepatitis and renal failure.40 It has been estimated that AHS complicates between 0.1% and 0.4% of allopurinol treatment courses, although rates have been estimated to be as low as 1 in 56,000 allopurinol users.44 AHS appears to be substantially more common in individuals positive for HLA-B*5801. In a small case-control study, all patients who developed AHS were positive for HLA-B*5801 compared with just 13% of allopurinol-treated patients without AHS.45 This risk allele is seen in approximately 2% to 7% of whites, 7% of blacks, and 8% of Asian Indians.46 Given the potential severity of AHS, patients should be educated about the remote possibility of this adverse event and cautioned to discontinue allopurinol with development of rash, particularly if this is accompanied by fever or mucocutaneous lesions.
Given the rare occurrence of AHS, allopurinol is generally well tolerated, with estimates suggesting that less than 5% to 10% of those exposed are intolerant to the drug.47 Rebound flares are among the most common adverse event accompanying allopurinol and other ULTs—an issue that is most prominent in the early phases of drug initiation and can be mitigated by anti-inflammatory prophylaxis. Isolated maculopapular skin rash can occur outside the context of AHS and is estimated to complicate approximately 1% to 3% of allopurinol treatment courses. Other common adverse events associated with allopurinol use are summarized in Table 65-1. Liver function abnormalities can be seen in approximately 6% to 7% of allopurinol users,20 although rates of severe liver injury appear to be exceedingly rare. The role and recommended frequency for laboratory surveillance in toxicity monitoring have not been well defined.
Fertility, Pregnancy, and Lactation
Although no human studies have investigated its use in pregnancy,48 allopurinol is classified as a Pregnancy Category C agent (animal reproduction studies have shown an adverse effect on the fetus without adequate human studies). Both allopurinol and oxypurinol are expressed in breast milk, and because drug effects on the developing infant are largely unknown, it should be administered to a nursing mother with caution.
Drug Interactions and Contraindications
Allopurinol drug interactions have been well characterized. Azathioprine and 6-mercaptopurine (6-MP) are metabolized primarily by XO, hence co-administration of allopurinol results in marked increases in circulating drug levels that can lead to bone marrow suppression.49–51 Theophylline is also metabolized by XO; therefore co-administration of this agent with allopurinol can lead to increased theophylline levels and can potentiate toxicity. Co-administration of allopurinol with ampicillin/amoxicillin has been associated with a higher incidence of drug-related rash.52 Thiazide diuretics may also reduce the renal excretion of allopurinol and oxypurinol, and it has been suggested that this could potentiate drug-related toxicity.53 Uricosurics increase the renal excretion of oxypurinol, thus offsetting to some degree the urate-lowering effect of allopurinol treatment.54 Allopurinol co-administration may increase drug levels of cyclosporine and warfarin, mandating close monitoring of drug levels and bleeding parameters, respectively. Other allopurinol-associated drug interactions are summarized in Table 65-1. Although regimens for desensitization have been described, allopurinol should be avoided in patients with known allergies such as AHS.
Febuxostat
Key Points
Febuxostat is a potent inhibitor of XO with a chemical structure that is distinct from allopurinol.
Role in Rheumatic Disease and Indications
Febuxostat is approved for the treatment of hyperuricemia in patients with gout; similar to all other ULTs, febuxostat is not indicated for the treatment of asymptomatic hyperuricemia. Given its unique structure, febuxostat represents an important alternative means of XO inhibition, particularly in gout patients intolerant to allopurinol.47 The role of febuxostat in gout management was recently addressed by an international panel, which provided guidance specific to indications, contraindications, monitoring, and issues requiring future research.55
Chemical Structure and Mechanism of Action
In contrast to allopurinol, febuxostat is a nonpurine analogue that reduces serum and urinary urate concentrations through potent and selective XO inhibition (see Figures 65-1 and 65-2). In contrast to allopurinol, which may inhibit other enzymes involved in purine and pyrimidine synthesis, febuxostat demonstrates significant enzymatic inhibition only for XO at therapeutic concentrations.56
Pharmacology
Following oral administration, febuxostat is rapidly absorbed from the GI tract with approximately 50% absorption, reaching peak plasma concentrations within a few hours57 with near complete plasma protein binding (see Table 65-1). Febuxostat displays linear pharmacokinetics that are not time dependent with drug metabolism occurring primarily in the liver through conjugation via uridine diphosphate glucuronosyltransferase (UGT) and oxidation via cytochrome P450 enzymes.57 Peak urate-lowering effects with febuxostat generally occur during the first 5 to 7 days of treatment. Drug elimination occurs via both hepatic and renal pathways. Although active metabolites are produced via oxidation, these are present in much lower plasma concentrations.
Dose and Drug Administration
Febuxostat is available in 40-mg (United States), 80-mg (United States and Europe), and 120-mg (Europe) tablets for oral administration, with usual dosing ranging from 40 mg to 120 mg daily (see Table 65-1). Febuxostat should be initiated at a lower dose (40 mg to 80 mg daily) and increased to higher doses (80 mg to 120 mg daily) if serum UA remains greater than 6.0 mg/dL after 2 weeks. In a phase II study, serum UA less than 6.0 mg/dL was obtained by 56%, 76%, and 94% of patients receiving 40 mg, 80 mg, and 120 mg per day of febuxostat, respectively, compared with 0% in the placebo-treated group.15 Mean serum UA reductions were greater with higher daily doses, ranging from a 37% reduction in the 40 mg/day group to 59% in the 120 mg/day group. Two subsequent trials lasting 28 weeks (n = 1067)18 and 52 weeks (n = 762)16 compared febuxostat (80 mg to 240 mg per day) with fixed-dose allopurinol (300 mg daily); both trials used the primary outcome of obtaining a serum UA less than 6.0 mg/dL at the last three consecutive monthly observations. The primary endpoint was achieved by 48% to 53%, 62% to 65%, and 69% of gout patients receiving daily doses of 80 mg, 120 mg, and 240 mg, respectively, compared with 21% and 22% of patients receiving fixed-dose allopurinol. Secondary outcomes in the trial by Becker and associates16 showed a decline in gout flare rates and an approximately 70% to 80% reduction in gout tophus area over follow-up—differences that were not significantly different from those observed with allopurinol. It is noteworthy that several studies of febuxostat16,18,20 employed fixed-dose allopurinol as an active comparator. As detailed previously, current gout treatment guidelines recommend initiating low-dose allopurinol (i.e., 100 mg/day) with escalations in dosing as needed to achieve target urate thresholds.4,14 Because optimal allopurinol dosing strategies were not used in these studies, these results likely overestimate the effectiveness of febuxostat relative to optimally dosed allopurinol.
Metabolized primarily in the liver, febuxostat may not require renal dosing.58 This receives support from a few small short-term pharmacokinetic studies that included a small number of patients with renal impairment.59,60 Only limited data have been obtained from longer-term studies of febuxostat in patients with moderate renal impairment (serum creatinine, ≈1.6 to 2.0 mg/dL), and essentially no data are available from patients with more severe renal dysfunction (serum creatinine >2.0 mg/dL). Available data, albeit limited, suggest that those with mild or moderate renal impairment (estimated creatinine clearance [CrCl] between 30 and 90 mL/min) experience similar efficacy and similar rates of toxicity compared with those with preserved renal function receiving equivalent doses of febuxostat.16,20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree