5 Muscle
Anatomy, Physiology, and Biochemistry
Approximately 660 skeletal muscles support and move the body under the control of the central nervous system. They constitute up to 40% of adult human body mass. Most skeletal muscles are fastened by collagenous tendons across joints in the skeleton. The transduction of chemical energy into mechanical work by muscle cells leads to muscle shortening and consequent movement. A high degree of specialization in this tissue is evident from the intricate architecture and kinetics of intracellular membrane systems, the contractile proteins, and the molecular components that transmit force extracellularly to the basement membrane and tendons. Muscle cells normally exhibit wide variations in activity level and are able to adapt in size, isoenzyme composition, membrane organization, and energetics. In pathologic states, they often become deconditioned. These examples of plasticity can be surprisingly swift and extensive. This chapter outlines the structure and function of muscle and its relationship to associated connective tissue. It also introduces the basis for the highly adaptive response to altered functional demands and diseases. Two excellent Web sites can be accessed for further information in these areas.1,2
Structure
Muscle Tissue
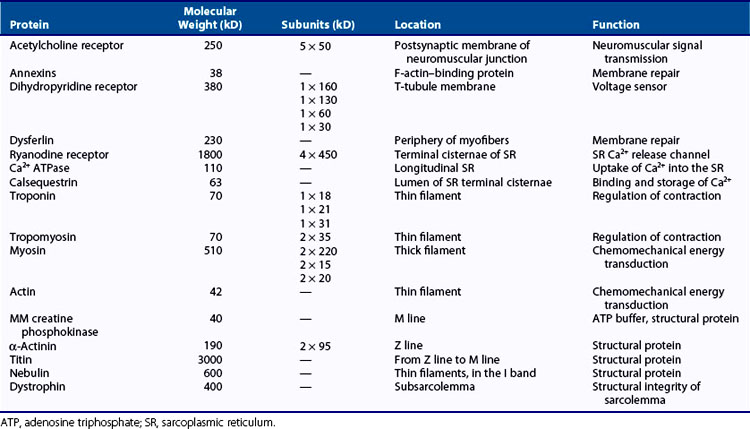
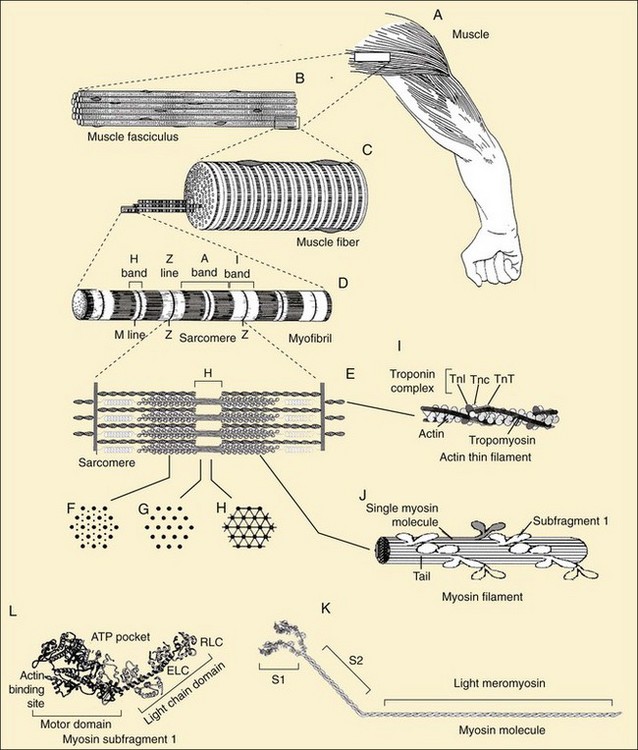
Parallel, aligned bundles of skeletal muscle fibers make up approximately 85% of muscle tissue and consist of a variety of signaling and contractile proteins (Table 5-1). Nerves, blood supply, and connective tissue structures that provide support, elasticity, and force transmission to the skeleton (see later discussion) constitute the remaining volume. Muscle fibers range in length from a few millimeters to 30 cm and in diameter from 10 to 500 µm, with a typical length of 3 cm and diameter of 100 µm. This elongated shape is determined by the organization of the contractile proteins that occupy most of the sarcoplasm. Each muscle has a limited range of shortening that is amplified into large motions by lever systems of the skeleton, usually operating at a mechanical disadvantage. Variations in geometric arrangements of the fibers—parallel, convergent (fan-shaped), pennate (feather-like), sphincter (circular), or fusiform (thick in the middle with tapered ends)—determine some of the mechanical properties. For example, a muscle with fibers aligned parallel to the force-generating axis will have more basic contractile units (i.e., sarcomeres as discussed later) in series than a similarly sized pennate muscle, allowing the parallel muscle to contract quicker, but with less force, than the pennate muscle. Muscles designed for strength (e.g., gastrocnemius) are typically pennate, whereas those designed for speed (e.g., biceps) tend to have parallel fibers. Muscles are commonly arranged around joints as antagonistic pairs facilitating bidirectional motion. When one muscle (the agonist) contracts, another (its antagonist) is relaxed and passively extended. Their roles reverse to actively generate the opposite motion, unless it occurs passively by the force of gravity.
An extensive network of areolar connective tissue, forming the endomysium, surrounds each muscle fiber. Fine nerve branches and small capillaries, necessary for the exchange of nutrients and metabolic waste products, penetrate this layer. The endomysium is continuous with the perimysium, a connective tissue network that ensheathes small parallel bundles of muscle fibers known as fasciculi, intrafusal fibers, larger nerves, and blood vessels. The epimysium encompasses the whole muscle. All three layers of connective tissue contain collagen, mostly types I, III, IV, and V, with types IV and V predominating in the basement membranes surrounding each skeletal muscle fiber. The α12α2-chain composition of the collagen IV isoform is the most prevalent and provides the mechanical stability and flexibility of the basil lamina3,4 (see Chapter 2). The perimysium and endomysium merge at the junction between muscle fibers and tendons, aponeuroses, and fasciae. These layers give the attachment sites great tensile strength and distribute axial force into shear forces over a larger surface area.
Fiber Types
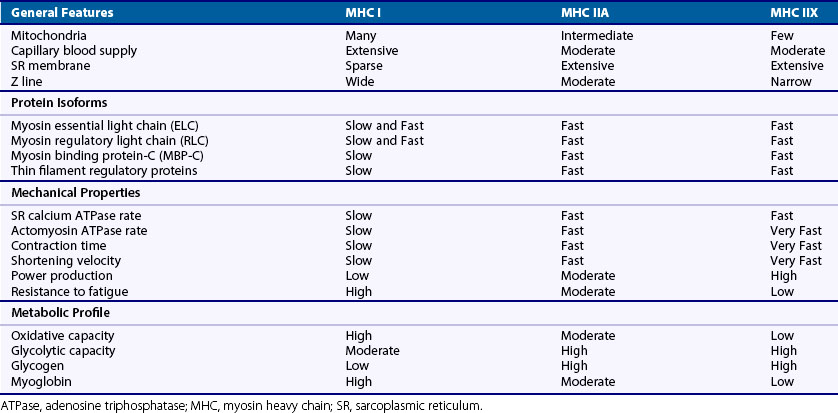
Muscles adapt to their specific functions. In any given muscle, part of this adaptation arises from its composition and organization of fiber types. Human skeletal muscle fibers can be classified according to their myosin heavy chain (MHC) isoform (I, IIA, or IIX). MHC molecules break down adenosine triphosphate (ATP) to produce the energy necessary for muscle contraction. The rate of ATP breakdown, or adenosine triphosphatase (ATPase) rate, of the MHC isoforms is I < IIA < IIX, leading to contractions that are relatively slow in MHC I fibers, fast in MHC IIA fibers and very fast in MHC IIA fibers. ATP synthesis primarily occurs through aerobic respiration (oxygen required) in MHC I or slow oxidative fibers; this is aided by their having more mitochondria, an increased capillary blood supply, and more myoglobin compared with MHC IIX, or fast glycolytic fibers that use anaerobic respiration (oxygen not required) to restore their ATP levels. MHC I fibers produce less power than MHC IIX fibers but are more resistant to fatigue. MHC IIA or fast oxidative-glycolytic fibers can use aerobic and anaerobic respiration and fall in between I and IIA fibers in terms of mitochondria, blood supply, myoglobin, power output, and fatigability. Although most human skeletal muscles contain a mixture of fiber types, MHC I fibers are more prominent in muscles used for posture and endurance, and MHC IIX fibers dominate in muscles used for quick movements of short duration. Notably, individual fibers can contain a mixture of MHC isoforms, leading to six different fiber types in humans (“pure”: MHC I, IIA, IIX; “mixed”: MHC I/IIA, IIA/IIX, I/IIA/IIX), which allow for a wide range of contractile properties. A list of various attributes of MHC isoforms can be found in Table 5-2.
During development, fiber-type specificity may be partially determined before innervation.5 Although the biologic events and signals responsible for designating functional specialization in muscle fibers are not fully understood, classic cross-innervation experiments demonstrated that innervation can dynamically specify and modify the type of muscle fiber.6 After cross-innervation, the functional and histologic properties listed in Table 5-2 shift toward the target fiber type over a few weeks’ time, indicating the ability of muscles to adapt and remodel in accordance with the pattern of neuronal activity.
Events during Muscle Contraction
Neural Control
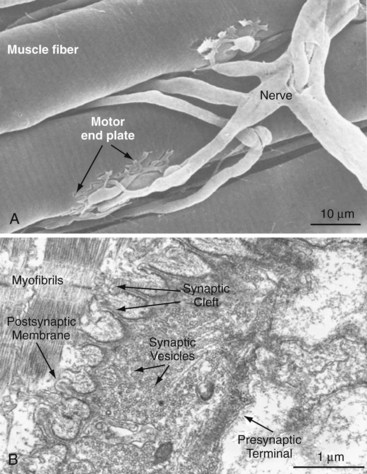
Voluntary control of muscle activation is a complex process. Afferent neurons emanating from sensory organs, such as cutaneous mechanoreceptors and thermoreceptors, pain receptors, joint receptors, and tendon organ and muscle spindles, provide the central nervous system (CNS) with stimuli in the form of action potentials that, with or without additional stimuli from the brain, provide the necessary information for feedback control of effector organs via efferent neurons.7 Efferent neurons are specifically called motor neurons if their axons innervate muscle. Many times, more afferent than efferent neurons afford effective feedback control of movement and posture. The afferent and efferent neurons are accompanied by Schwann cells, which are glial cells residing in the peripheral nervous system.8 Neurons are termed myelinated if Schwann cells wrap around the axon at regularly spaced intervals. The points of bare axon between these Schwann cells are called nodes of Ranvier. Myelination enhances the velocity of action potential propagation by compelling a saltatory conduction of the action potential between neighboring nodes. Schwann cells may also fully or nearly fully cover the axon, thus rendering the neuron unmyelinated and relatively slow in action potential propagation. Three groups of myelinated motor neurons (α, β, γ) are distinguished by diameter, propagation velocity, and target fiber type. Skeletal muscle fibers typically are innervated at several neuromuscular junctions along their length by branches of an α-motoneuron (largest and fastest) or β-motoneuron (Figure 5-1). Muscle spindles are innervated by β- or γ-motoneurons, in addition to the afferent system, for sensing muscle length and force. A single motor neuron and the muscle fibers it innervates constitute a motor unit. When a motor neuron is excited, all fibers in the motor unit are triggered to contract simultaneously. A motor unit responsible for fine movement contains few muscle fibers, but motor units for gross movement generally contain many fibers. The level of muscle activation is controlled from the CNS by the number of motor units recruited and the stimulus rate.9 Stimulus rate can be so infrequent as to elicit single muscle twitches, such as occur with the monosynaptic stretch reflex involving patellar tendon stretch and quadriceps activation, or conversely so frequent that individual twitches effectively fuse, causing nearly continuous activation of muscle force.10
Neuromuscular Transmission
At the neuromuscular junction, the axon tapers, loses its myelin sheath, and ends as a presynaptic terminal crowded with vesicles containing the neurotransmitter acetylcholine. The postsynaptic membrane of the muscle is indented into folds that increase its surface area and the number of nicotinic acetylcholine-receptors bound therein (see Figure 5-1). The junctional cleft is a 20- to 40-nm-wide space between the presynaptic and postsynaptic membranes.11 When the motor neuron action potential reaches the presynaptic terminal, local voltage-gated Ca2+ channels open and extracellular Ca2+ streams into the terminal. Within milliseconds of Ca2+ influx, the acetylcholine-loaded vesicles fuse with the presynaptic membrane.12 Exocytosed acetylcholine rapidly diffuses across the junctional cleft and binds to the nicotinic acetylcholine receptors, which in turn open Na+ and K+ channels of the postsynaptic membrane. The membrane is locally depolarized. An action potential is initiated and propagates along the muscle membrane (sarcolemma) at velocities up to 5 m/sec.
Excitation-Contraction Coupling
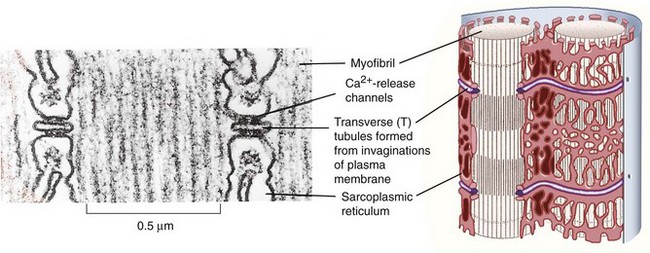
A network of tubules invaginate the sarcolemma and run deep into the muscle fiber. This transverse tubule network (T-tubules) pervades the fiber at regular intervals coinciding with sarcomere boundaries along the length of the muscle and surrounds the contractile apparatus with connected longitudinal and lateral segments (Figure 5-2). The lumen of this network is open to the extracellular space, and it contains high Na+ and low K+ concentrations of interstitial fluid.13 Action potentials at the surface membrane invade the entire T-tubular system. A specialized type of endoplasmic reticulum forms an entirely intracellular membrane system termed the sarcoplasmic reticulum (SR). Prevalent structures containing a T-tubule flanked by two terminal cisternae of the SR to form junctional complexes are termed triads (see Figure 5-2). Terminal cisternae contain oligomers of the Ca2+-binding protein calsequestrin that provide the fiber with an internal reservoir of calcium ions. Ca2+ channels, termed dihydropyridine receptors (DHPRs), are localized in the T-tubule membranes facing the cytoplasmic domain of SR Ca2+ release channels, also called ryanodine receptors (RyRs), in the terminal cisternae membranes.14 These membrane proteins are further characterized in Table 5-1.
When an action potential depolarizes the T-tubular membrane, the DHPRs, primarily voltage sensors in skeletal muscle, transfer a signal from the T-tubules to the RyRs by direct interprotein coupling. Ca2+ is then released cooperatively through the RyRs from the SR into the myoplasm, where it activates the contractile machinery.15 This sequence of events is termed excitation-contraction coupling.
Mutations in the α-subunit of the DHPR in dysgenic mice lead to paralysis, because in these mutants, depolarization of the skeletal muscle membrane does not initiate release of Ca2+ from the SR. Excitation-contraction coupling can be restored in cultured cells from these mice by transfection with complementary DNA encoding for the DHPR,16 and transfections using chimeric constructs17 have pinpointed the domain within the DHPR that specifies skeletal- or cardiac-type excitation-contraction coupling.18 Isoforms of the RyRs also help determine the characteristics of coupling between T-tubules and the SR.19 Channelopathies in human skeletal and heart muscle have been linked to DHPR mutations.20,21 Human malignant hyperthermia occurs in individuals with mutant RyRs that become trapped in the open state after exposure to halothane anesthetic agents.22
Contractile Apparatus
The specific locations and functions of the contractile proteins are listed in Table 5-1. Myofibrils (Figure 5-3D) are long, 1-µm-diameter cylindrical organelles that contain the contractile protein arrays responsible for work production, force generation, and shortening. Each myofibril is a column of sarcomeres, the basic contractile units, which are approximately 2.5 µm in length and are delimited by Z lines (Figure 5-3D and E) containing the densely packed structural protein α-actinin. The contractile and structural proteins within each sarcomere form a highly ordered, nearly crystalline lattice of interdigitating thick and thin myofilaments23 (Figure 5-3E, I, and J). Myofilaments are remarkably uniform in both length and lateral registration, even during contraction,24 resulting in the cross-striated histologic appearance of skeletal and cardiac muscles. This highly periodic organization has facilitated biophysical studies of muscle by sophisticated structural23 and spectroscopic techniques.25,26
Thick filaments (1.6-µm long) containing the motor protein myosin are located in the center of the sarcomere in the optically anisotropic A band (Figure 5-3D). These thick filaments are organized into a hexagonal lattice stabilized by M protein27 and muscle-specific creatine phosphokinase28 in the M line (Figure 5-3D and E). Myosin (Figure 5-3K) is a highly asymmetric 470-kD protein containing two 120-kD globular NH2-terminal heads, termed cross-bridges or subfragment-1 (S1) (Figure 5-3L), and an α-helical coiled-coil rod, light meromyosin (Figure 5-3K). Two light chains, essential and regulatory, ranging from 15 to 22 kD, are associated with the heavy chain in each S1 (Figure 5-3L). The rod portions of approximately 300 myosin molecules polymerize in a three-stranded helix to form the backbone of each thick filament (Figure 5-3J). The cross-bridges, protruding from these backbones, contain ATPase and actin-binding sites responsible for the conversion of chemical energy into mechanical work. Besides their role in muscle contraction, at least 20 classes of nonmuscle myosins accomplish diverse tasks in cell motility such as chemotaxis, cytokinesis, pinocytosis, targeted vesicle transport, and signal transduction.29 Thus myosin is the target for mutations leading to a number of inherited muscle and neurologic diseases.30,31
Thin filaments (Figure 5-3I) are double-stranded helical polymers of actin that extend 1.1 µm from each side of the Z line and occupy the optically isotropic I band (Figure 5-3D and E). A regulatory complex containing one tropomyosin molecule and three troponin subunits (TnC, TnT, and TnI) is associated with each successive group of seven actin monomers along the thin filament (Figure 5-3I).23 In the region where the thick and thin filaments overlap, the thin filaments are positioned within the hexagonal lattice equidistant from three thick filaments (Figure 5-3F). Both sets of filaments are polarized. In an active muscle, an interaction between the two filaments causes a concerted translation of the thin filaments toward the M line that shortens the sarcomere, and thus the muscle fiber and whole muscle (Figure 5-3A through D). Actin is ubiquitous in the cytoskeleton of eukaryotic cells and, like myosin, fulfills many roles in determining cell shapes and motions.32,33 Control of the actin cytoskeleton and diseases due to mutations in actin-binding proteins are being intensively investigated.34
Two of the largest identified muscle proteins, titin and nebulin, function in assembly and maintenance of the sarcomeric structure. Individual titin molecules (≈3000 kD) are associated with the thick filament and extend from the M line to the Z line.35 Titin contains repeating fibronectin-like immunoglobulin and unusual proline-rich domains that confer molecular elasticity on the resting sarcomere.36 Nebulin (≈800 kD) is associated with the Z line and thin filaments.35 Protein connections from the contractile apparatus through the sarcolemma to the extracellular matrix are described later in this chapter. The cytoskeleton of muscle fibers also contains cytoplasmic actin, microtubules, and intermediate filaments.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree