100 Treatment of Osteoarthritis
Osteoarthritis (OA) is the most common form of arthritis.
Pain is the most common symptom in patients with OA.
Currently available pharmacologic interventions are directed at symptomatic relief.
Investigation continues into potential disease-modifying interventions in OA.
![]() Video available on the Expert Consult Premium Edition website.
Video available on the Expert Consult Premium Edition website.
OA can be defined radiographically or clinically. The most useful definition, however, includes symptoms and radiographic changes. If a purely radiographic definition is used, it can be demonstrated that almost all individuals older than 75 years have OA.1 Although the epidemiology of OA is well covered in Chapter 99, it has been estimated that between 10% and 30% of those affected with OA are significantly disabled, making OA the leading cause of chronic disability in the United States.2 This leads to substantial direct and indirect costs.
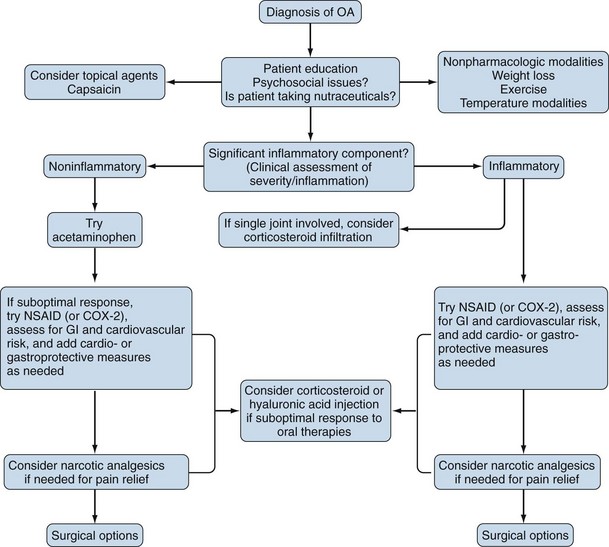
Traditional treatment paradigms for OA have conceded the inexorable progression of the disease and concentrated on pain management.3 A simplistic but potentially useful algorithm is provided in Figure 100-1. As the population ages, there will be increasing societal pressure on physicians, particularly rheumatologists, to improve the available treatments for OA.4–6 Researchers have turned to the investigation of agents that might delay the progression of OA. Particular investigational agents have included collagenase inhibitors, nutritional supplements, and polysaccharides, although many novel molecular entities are now under exploration.7
Management
The management of OA can be divided into nonpharmacologic interventions (Table 100-1), pharmacologic interventions, and surgical options. Pharmacologic interventions can be further subdivided into symptomatic therapy and potential structure- or disease-modifying therapy.
Table 100-1 Nonpharmacologic Management of Osteoarthritis
| Conventional Options |
| Unconventional Options |
Nonpharmacologic Interventions
Psychosocial Interventions
Some patients may develop significant emotional disturbances related to the pain and changes in normal daily activities that can stem from OA. These may include mood disorders such as depression or sleep disorders. Worsened measures of mental health have been associated with increased OA pain and risk of flares.8 Suspicion of either condition should lead to an evaluation by a psychiatrist or a primary physician who regularly manages these types of disorders.
Weight Loss
Obesity is an important risk factor in the development of OA of the knee.9,10 Further, higher body mass index (BMI) has been associated with an increased risk of progression of OA of the knee.11 This can be compounded by malalignment—namely, varus and valgus deformities that modulate the effect of weight on knee OA.12 In one study, BMI was associated with OA severity in those with varus deformity but not in those with valgus.
Regimens of weight loss and exercise have been associated with improvement in pain and disability in OA of the knee.13 Weight loss alone has been associated with a decrease in the odds of developing symptomatic knee OA.14 One study suggested that a reduction in the percentage of body fat, rather than weight, may be significant in reducing pain from OA of the knee.15 The symptom-relieving effects of weight loss have been shown to last as long as 1 year.16 The combination of weight loss and exercise can be superior to either intervention alone.17
Temperature Modalities
Topical applications of heat or cold can be a helpful adjunct to the therapeutic plan. These are more effectively used in superficial joints such as the knees than in deep ones such as the hip. An acute injury such as a sprained ankle calls for cold applications for the first 2 to 3 days.18 In a setting of chronic pain, most patients prefer warm applications, although if superior pain relief is obtained from cold applications, these can be continued.
Warm applications can be in the form of warm soaks or heating pads. Individual sessions should not exceed a temperature of 45° C or last more than approximately 30 minutes.19 The application of warmth should be avoided over certain areas such as close to the testicles and in patients with poor vascular supply, neuropathy, or cancer. Benefits of warm applications include decreased pain and stiffness, along with relief of muscle spasm and prevention of contractures.
Exercise
Periarticular structures, particularly muscles, influence the expression of OA. This is likely due to their role in providing stability to the joints and in dampening some of the forces acting across joints. Quadriceps muscle weakness has been postulated as a risk factor for OA of the knee.20 Quadriceps strengthening exercises have been advanced as fundamental to the management of conditions such as chondromalacia patellae.21
Both the dynamic and isometric exercise arms of a 16-week study of patients with knee OA showed equivalent improvement in symptoms and physical functioning.22 Walking can be beneficial, and supervised fitness-walking regimens can improve function in those with OA of the knee.21 Home-based exercise interventions also significantly improve symptoms in those with knee OA.23,24 Finally, community-based aquatic exercise programs such as aquatic aerobics have merit.25
Orthotics and Bracing
Orthotics—ranging from insoles to braces—can be effective in providing symptomatic relief and are probably underused by most physicians. Studies have demonstrated that lateral wedged insoles provide substantial relief to those with medial compartment knee OA, particularly those with varus deformity.26 In some studies, those with milder symptoms obtained greater benefit.27 Knee braces have been evaluated as well. Valgus bracing of patients with medial compartment OA can reduce pain and increase levels of activity.28 In one study, medial taping of the patella reduced the pain of those with patellofemoral compartment OA by 25%.29
Heel lifts have been tried in those with hip OA. In an uncontrolled study, most patients reported diminished symptoms. Time to improvement lengthened with the radiographic stage of OA.30 For those with calcaneal spurs or foot joint OA in general, appropriate athletic-type footwear is recommended. A good athletic shoe should provide medial arch support and calcaneal cushioning, as well as good mediolateral stability.
Those with carpometacarpal joint arthritis should initially be offered conservative management including the use of splints. In one trial, 70% of patients treated with a 7-month intervention that included the use of splints were able to improve their symptoms considerably and avoid surgical intervention.31
Cane/Walking Aid
The appropriate use of a cane (walking stick) can be an important adjunct, particularly in OA of the hip. It has been estimated that a cane can provide up to a 40% reduction in hip contact forces during ambulation.32 The cane should be used in the hand contralateral to the affected hip or knee33 and should be advanced with the affected limb while walking. The appropriate cane size is that which results in about a 20-degree flexion of the elbow during use.34 A useful approximation is a cane that is equal to the distance from the floor to the patient’s greater trochanter.
Other Interventions
Studies of transcutaneous electrical nerve stimulation (TENS) have generally been small. A review of TENS studies in OA of the knee concluded that a trend toward symptom improvement existed, warranting larger, well-controlled studies.35 In one randomized, controlled study, patients had initial symptom reduction, but at 1-year follow-up, only two patients continued to use the device.36 TENS use for 3 weeks was compared with three weekly hyaluronic acid injections in 60 patients with OA of the knee. Pain relief was observed in both groups through the 6 months of follow-up. There was superior improvement in the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) physical function subscale score for the hyaluronic acid group.37
Pulsed electromagnetic fields have been tested in double-blind, placebo-controlled trials. These fields are applied through the daily use of a brace-type device. In one study, a primary endpoint of pain reduction was not achieved.38 Another study did not meet its primary endpoint but reported an improvement in knee stiffness in subjects younger than 65 years, without an accompanying reduction in pain.39
The use of static magnets in chronic knee pain has become popular with some patients. In one double-blind, randomized, placebo-controlled trial of 43 patients, the WOMAC pain and physical function subscales, along with a 50-foot walk, demonstrated a statistically significant benefit of static magnets at 2 weeks.40 Another 29-patient double-blind, placebo-controlled trial in knee OA reported a benefit over placebo after 4 hours of use, but there were no significant differences between groups at 6 weeks of continued treatment.41 The potential mechanism for any effect remains unclear, and larger, longer-term studies are necessary before any clinical benefit can be postulated.
Acupuncture is being formally tested in a National Institutes of Health (NIH)–sponsored multicenter clinical trial. It has been difficult to develop appropriate controls to test acupuncture’s clinical efficacy. Most recent studies have tried to employ “sham” methods in the control arm such as the use of blunted, telescopic needles.42 Early clinical trials43,44 and one literature review45 concluded that acupuncture shows promise in the treatment of knee pain from OA. A double-blind, randomized, placebo-controlled trial of acupuncture as adjunctive therapy in OA of the knee enrolled 570 patients in two outpatient clinics. Reduction in knee pain in the true acupuncture group was superior to that in the sham acupuncture group at 26 weeks by WOMAC function score, WOMAC pain score, and patient global assessment. Twenty-five percent of the patients in each of the acupuncture groups were unavailable for analysis at 26 weeks, however.46
Spa therapy also has advocates. It has been touted for low back pain and for lower extremity OA.47 However, randomized, controlled studies are lacking.48 Yoga has also shown some symptomatic benefit in OA of the hands on the basis of limited testing.49
Pharmacologic Interventions
Topical Agents
Topical agents for the management of OA are available without a prescription in the United States (Table 100-2). The two most widely used types are preparations containing capsaicin and those containing topical NSAIDs.
Table 100-2 Symptom-Relieving Pharmacologic Therapies for Osteoarthritis
| Topical |
| Systemic |
| Intra-articular |
NSAID, nonsteroidal anti-inflammatory drug.
Capsaicin is a pungent ingredient found in red peppers (such as hot chili peppers). The mechanism of action is thought to be through selective stimulation of unmyelinated type C afferent neurons, causing the release of substance P. This release reversibly depletes the stores of substance P, a neurotransmitter of peripheral pain sensations.50 Capsaicin preparations are available in concentrations of 0.025% or 0.075% in either ointment or, more recently, “roll-on” form, and they can be applied up to four times daily. They have been tested in controlled, double-blind studies in OA of the hands and knees.51,52 Patient response is quite variable, with some obtaining significant pain relief and others not being able to tolerate the burning or stinging sensation produced by its application. Usually, the counterirritant sensation decreases gradually with repeated use, but pain relief remains. Although safe overall, capsaicin products can be irritating if they come in contact with mucosal surfaces, particularly the eyes. Patients should wear disposable gloves, if possible, when applying the agents. There may be some reddening of the skin where the compound is applied.
Topical NSAID preparations are popular worldwide for the treatments of OA.53,54 Safety concerns about traditional oral NSAIDs were the driving force in the use of these topical agents,55 although questions remain as to their absorption and the degree of relief obtained. Results of placebo-controlled trials in OA of the knee have been conflicting. Some demonstrated symptomatic relief with topical application of gels containing NSAIDs such as diclofenac,56,57 whereas others showed only trends favoring the NSAID or no difference at all. In one trial, diclofenac gel was compared with placebo in 238 patients with OA of the knee over 3 weeks. The primary outcome was average pain with movement on days 1 to 14. The group on diclofenac gel had statistically superior improvement in this variable compared with those on placebo. WOMAC scores for function, pain, and disability were also significantly superior to placebo at weeks 2 and 3.58 Transdermal diclofenac patches are also available and can be applied twice daily to painful articular locations. Patients were also randomized to receive eltenac or placebo gel over 4 weeks. Eltenac is a nonselective NSAID that is structurally similar to diclofenac. The primary endpoint was global pain on a visual analogue scale (VAS). At 4 weeks, there was a trend, but no statistical difference, favoring the eltenac gel. Two patients in the active treatment group and two in the placebo group had local itching, reddening, or both in the application area. There are also menthol- and salicylate-based over-the-counter topical preparations, but there are no published trials supporting their use in OA. Finally, lidocaine has gained popularity as a topical agent for musculoskeletal pain. Transdermal lidocaine 5% patches are available for management of pain and can be applied to up to three articular and periarticular locations at a time as an analgesic agent for 12-hour periods.
Systemic Agents
Non-narcotic Analgesics.
Acetaminophen (paracetamol) has often been touted as the initial systemic intervention for the management of OA. This is mainly due to its favorable side effect profile but also to a perception of its equivalent efficacy to NSAIDs. This perception derives from studies of OA in which patients were not stratified in terms of degree of symptoms. In one study, acetaminophen 4 g/day was equivalent to ibuprofen 1200 or 2400 mg/day, with the notable exception of pain at rest.59 A meta-analysis of 10 randomized, controlled trials concluded that acetaminophen is effective in the relief of pain associated with OA. However, the effect was small, and there was no improvement in overall WOMAC score. This suggests that acetaminophen may be effective for the relief of pain and should not be expected to have a strong effect on stiffness or function.60 More recently, it has been noted that NSAIDs may have superior efficacy in patients with more symptomatic or inflammatory presentations because acetaminophen has no anti-inflammatory effects at approved doses.61 A recent database review concluded that the available evidence suggests that NSAIDs have superior efficacy in symptomatic relief in those with hip or knee OA and also in those with moderate to severe levels of pain from OA.62 Particular concerns in patients taking acetaminophen include the concomitant use of alcohol or over-the-counter products containing acetaminophen. Either of these situations can lead to the possibility of hepatic toxicity through toxic metabolites.
Nonsteroidal Anti-inflammatory Drugs.
NSAIDs are the most commonly prescribed medications for the treatment of OA. Nonselective NSAIDs work through nonspecific inhibition of cyclooxygenase isoforms 1 and 2 (COX-1 and COX-2). COX-1 is constitutively expressed in renal and gastrointestinal (GI) tissues, among others. COX-2 is inducible in inflammatory responses. The major side effects of NSAIDs are GI toxicities (gastritis, peptic ulcer disease) and renal toxicities (interstitial nephritis, prostaglandin inhibition–related renal insufficiency). Because GI tissues have a higher expression of COX-1, a selective COX-2 inhibitor might spare patients the GI side effects. Unfortunately, COX-2 is expressed in renal tissue, and COX-2–specific drugs such as traditional NSAIDs have potential adverse renal effects. This is especially true in those with baseline renal insufficiency. Concerns about cardiovascular risks led to the voluntary withdrawal of rofecoxib from the market in the United States. There have also been concerns about celecoxib at a dose of 200 mg twice daily, owing to an increased relative risk for myocardial infarction in an adenomatous polyp trial63; this, however, has not been confirmed in six observational studies.64 All NSAIDs and COX-2–specific agents have received “black box” warnings in their package inserts addressing cardiovascular risk. Alternative mechanisms of action of NSAIDs such as interference with receptors in the cell membrane phospholipid bilayers have been proposed.65
To reduce the potential for adverse GI events, misoprostol can be added to the therapeutic regimen. It is a prostaglandin E2 analogue that has been shown to reduce the GI side effects of NSAIDs when used at 200 µg three times a day.66 Diarrhea is a potential side effect. The use of a concomitant proton pump inhibitor may reduce upper GI endoscopic ulceration rates from NSAIDs, although no study has attempted to show a decrease in events such as symptomatic ulcers or bleeds.67 Over-the-counter doses of H2 blockers and antacids have not been shown to reduce either endoscopic or serious clinical GI events. COX-2–specific inhibitors are the latest drugs used in an attempt to reduce the GI adverse event profile of OA therapy.
COX-2–specific inhibitors are highly selective for COX-2 in vitro. Currently, only one such agent is available in the United States, celecoxib. Others such as etoricoxib are available elsewhere. These agents have been shown to reduce the rate of endoscopic ulceration by more than 50% when compared with nonselective NSAIDs. Celecoxib significantly reduces the rates of symptomatic ulcers, bleeds, perforations, and obstructions in patients not concurrently on aspirin.68,69 It remains unclear how substantial the gastrointestinal benefits of these compounds are to patients taking aspirin. Because COX-2–specific agents can inhibit endothelial prostacyclin but do not affect platelet thromboxane, cardiovascular safety remains an area of investigation.70,71
Combination COX-lipoxygenase inhibitors are still investigational. It remains to be seen how these will compare with traditional NSAIDs and with COX-2 inhibitors in terms of both safety and efficacy.72 Animal studies have hinted at the possibility of a structure- and disease-modifying effect.73
Narcotic Analgesics.
Tramadol is an oral medication with mild suppressive effects on the mu opioid receptor. It also inhibits the uptake of norepinephrine and serotonin74 and is not thought to have significant addictive tendencies.75 It is available alone or in combination with acetaminophen and is not a controlled-schedule medication in the United States.76 Tramadol has been used for the symptomatic relief of OA.77 Seizures and allergic reactions are potential side effects.78 A warning of increased risk of suicide in certain patients, similar to that on antidepressants, has been added to the label. The incidence of nausea can be reduced by slowly escalating the dose until the desired pain relief is achieved.
One study compared tramadol and acetaminophen with the combination of codeine and acetaminophen.79 Patients with OA or chronic low back pain were randomized to receive tramadol and acetaminophen (37.5 mg and 325 mg, respectively) or codeine and acetaminophen (30 mg and 300 mg, respectively) for 4 weeks. Pain relief and changes in pain intensity were equivalent in both groups. Those on codeine and acetaminophen had a significantly higher incidence of somnolence (24% vs. 17%) and constipation (21% vs. 11%). The tramadol-acetaminophen combination also provides symptomatic relief as add-on therapy in OA patients receiving NSAIDs or COX-2 agents as baseline therapy.80
Extended-release narcotic analgesics have been tested in clinical trials in OA. This approach is intended to achieve a lower level of peak-to-trough variability in the plasma concentration of the narcotic. An extended-release, once-a-day preparation of tramadol relieves pain in OA of the knee and hip.81 Extended-release oxymorphone dosed twice a day also provides relief in those with moderate to severe pain from OA of the hip or knee, as demonstrated by a VAS and the WOMAC composite index, as well as the subscales for pain, stiffness, and physical function.82
Transdermal fentanyl, a narcotic analgesic, has been used in the treatment of moderately to severely symptomatic knee and hip OA. It relieved pain and improved function in clinical trials as judged by a VAS and the WOMAC physical function subscale.83
Intra-articular Agents
Corticosteroids.
Although there is no role for systemic corticosteroids in OA, local intra-articular corticoid preparations have a long history in the management of OA. Corticosteroids have been shown to downregulate the expression of adhesion molecules. This, in turn, can reduce cellular infiltration into the joint and subsequent inflammation. Corticosteroid injections slow macrophage-like cell infiltration of the synovium in OA.84 The dose of steroid injected is determined by the volume of the joint being injected, with larger joints such as the knee receiving higher doses. The risk of joint infection is low if proper technique is employed. Postinjection flares due to corticosteroid crystal synovitis can occur.
There is a relative dearth of information from clinical trials of intra-articular corticosteroid injections. However, in one study, symptomatic benefit from corticosteroid injection for OA of the knee was demonstrated in a double-blind trial at 1 and 4 weeks postinjection.85 Another trial attempted to assess the possible disease-modifying effects of corticosteroids by randomizing 68 patients to corticosteroid or saline injections of the knee every 3 months for 2 years. At the study’s end, there was no significant difference in rate of joint space narrowing; thus no case could be made for a disease-modifying effect of corticosteroid injections. There was a trend favoring pain relief in the corticosteroid group as measured by the pain subscale of the WOMAC.86 A review of published studies of intra-articular corticosteroid injections in OA concluded that the short-term symptomatic benefits have been well established, with few adverse events, but long-term benefits have not been confirmed.87
The specific corticosteroid compound used, the frequency of injections, and other factors related to the use of corticosteroid injections in OA vary widely and are heavily influenced by the training program the rheumatologist attended and where he or she practices.88 In general, corticosteroid injections are believed to be most effective in patients with evidence of inflammation, effusions, or both. Because of concerns over possible deleterious effects, usually no more than four corticosteroid injections per year are given in a particular joint. Further discussion of arthrocentesis can be found in Chapter 54.
Hyaluronic Acid Derivatives.
Synthetic and naturally occurring hyaluronic acid derivatives are administered intra-articularly. Although often mentioned as potential structure-modifying agents, these products are presently considered symptom-modifying drugs. Their molecular weights vary (from <100,000 to >1 million Svedberg units), depending on the preparation. They reportedly reduce pain for prolonged periods and may improve mobility.89 Improvement in overall physical functioning has also been reported.90 The mechanisms of action are not known. However, there is evidence of an anti-inflammatory effect (particularly at high molecular weight), a short-term lubricant effect, an analgesic effect by direct buffering of synovial nerve endings, and a stimulating effect on synovial lining cells, leading to the production of normal hyaluronic acid.91
Several preparations have been approved in the United States for OA of the knee. The injection regimens vary from five weekly injections to one injection depending on the product selected by the clinician for use.92 Pain relief has been the primary outcome in these studies with relief at 26 weeks postinjection for some. In one study, three weekly hyaluronic acid intra-articular injections provided comparable pain relief to a single corticosteroid intra-articular injection at 1-week follow-up; at 45 days’ follow-up, hyaluronic acid was superior to the corticosteroid.93 In a Canadian study, 102 patients with OA of the knee were randomized to three weekly intra-articular injections of hylan G-F (Synvisc), hylan G-F plus an NSAID, or NSAID alone. At 26 weeks, both groups receiving hylan G-F were significantly better than the group receiving NSAIDs alone.94
Substantial clinical responses to the saline injections used as placebo in hyaluronic acid trials have sometimes made data interpretation challenging. In a double-blind, placebo-controlled trial, 495 patients with knee OA were randomized to receive five intra-articular injections of hyaluronic acid (Hyalgan) given 1 week apart, placebo, or naproxen (500 mg orally twice a day) and followed for 26 weeks.95 Patients in the group receiving hyaluronic acid had significantly greater improvement in pain on the 50-foot walk compared with placebo, and more of them had a 20-mm or greater reduction in pain as judged by a VAS. At the conclusion of the trial, more hyaluronic acid–treated patients (47.6%) had slight pain or were pain free compared with placebo-treated (33.1%) or naproxen-treated (36.9%) patients. As expected, GI adverse events were significantly more common in the naproxen group than the hyaluronic acid and placebo groups.
Hyaluronic acid preparations have been tested in other randomized trials, with symptomatic relief of OA of the ankles, shoulders, and hips being reported.96–98 One multicenter, randomized, double-blind study, reported as an abstract, revisited the issue of disease modification with hyaluronic acid. Patients received three courses of three intra-articular knee injections of either hyaluronan or saline over the course of 1 year. Joint space width was assessed using standing, weight-bearing radiographs; 273 patients completed the trial and had complete data collection. This study failed to demonstrate a disease-modifying effect for hyaluronan therapy because the primary endpoint was not met. Both the active treatment group and the placebo group had similar joint space narrowing during the study period. In those with a joint space width of 4.6 mm or greater at entry, hyaluronan use led to slightly less joint space narrowing than saline (placebo, 0.55 mm ± 1.04; hyaluronan, 0.13 mm ± 1.05; P = .02).99 These results have not been confirmed in other trials. Hyaluronic acid products continue to be actively investigated in shoulder joint OA, periarthritis,100 and adhesive capsulitis.101
Nutraceuticals
Two nutritional supplements—glucosamine and chondroitin sulfate—have received significant attention (Table 100-3). Health food stores and the lay press rather dubiously proposed them as “cures for arthritis.” The mechanism of action of glucosamine sulfate is uncertain. Some in vitro experiments have shown stimulation of the synthesis of cartilage glycosaminoglycans and proteoglycans.102,103 Others have shown that glucosamine and N-acetylglucosamine inhibit interleukin (IL)-1β– and tumor necrosis factor (TNF)–induced nitric oxide production in normal human articular chondrocytes.104 N-acetylglucosamine also suppresses the production of IL-1β and stimulates IL-6 and COX-2.
Table 100-3 Nutraceuticals for Osteoarthritis
Glucosamine
Urinary excretion of glucosamine (and other glycosaminoglycans) has been investigated and found to be elevated in both OA and rheumatoid arthritis.105 Supplementation with glucosamine sulfate, an intermediate in mucopolysaccharide synthesis, has been tried both orally and intramuscularly as therapy for OA. Glucosamine sulfate (400 mg injected intramuscularly twice weekly for 6 weeks) reduced the severity of disease as judged by the Lequesne index when compared with placebo.106 A randomized, double-blind, parallel-group study in knee OA compared 500 mg oral glucosamine sulfate three times a day with 400 mg ibuprofen three times a day for 4 weeks. The response to ibuprofen was more rapid, but at 4 weeks, there was no statistically significant difference in the response rate (reduction of at least 2 points in the Lequesne index).107 No group in the study received higher, anti-inflammatory doses of ibuprofen. An NIH-sponsored trial is currently under way in the United States to more thoroughly study the symptom-relieving and possible structure-modifying properties of glucosamine. Meanwhile, some already advocate the use of glucosamine as part of the first line of therapy for symptomatic OA.108
Glucosamine has also been compared with acetaminophen in an industry-sponsored trial. In the GUIDE trial, 318 patients with knee OA were randomized to glucosamine sulfate soluble powder 1500 mg once a day, acetaminophen 1000 mg three times a day, or placebo for 6 months. The main efficacy parameter was the 6-month change in the Lequesne index. At 6 months, the glucosamine group achieved significantly greater efficacy versus placebo. Those on acetaminophen failed to achieve a statistically significant benefit versus placebo by either the Lequesne index or WOMAC. There was no statistically significant difference between those on glucosamine and those on placebo on the basis of WOMAC outcomes.109 Another clinical trial randomized 80 patients with knee OA to either glucosamine sulfate 1500 mg/day or placebo for 6 months. There was no difference between glucosamine and placebo in the primary variable of patients’ global assessment of pain in the affected knee.110 Another trial used a unique Internet-based recruiting system and followed 205 patients with knee OA randomized to glucosamine sulfate 1500 mg/day or placebo for 12 weeks.111 The primary endpoint was the pain subscale of the WOMAC. At study conclusion, there was no difference in the groups with regard to pain, physical function, or overall WOMAC scores. Stratification by severity of OA, glucosamine product used, or use of NSAIDs did not alter the results. The Cochrane review of glucosamine therapy in OA analyzed a pool of 20 studies and 2570 patients. Pain and function improved by 28% and 21%, respectively, by the Lequesne index, compared with placebo. There was no improvement in the overall WOMAC pain and function scales. There has been speculation that these inconsistencies in study results may be due to a lack of standardization in glucosamine preparations.112
A recent discontinuation trial has added to the uncertainty about glucosamine’s efficacy. It found that 137 patients who had been clinically classified as moderate responders to glucosamine sulfate were equally likely to experience an OA flare whether they continued or discontinued the glucosamine. No statistically significant differences between the groups were noted in pain and WOMAC function scores after 6 months.113
Combination products containing both glucosamine and chondroitin have become popular in the United States, despite a dearth of clinical trial data. One small, placebo-controlled trial randomized patients with knee OA to receive a regimen of glucosamine hydrochloride (1000 mg), chondroitin sulfate (800 mg), and manganese ascorbate (152 mg) twice a day or placebo.114 Patients were evaluated at baseline and then every 2 months for 6 months using the Lequesne index of OA severity. At 4 and 6 months, those with mild to moderate radiographic OA of the knee showed significant improvement by the Lequesne index compared with those on placebo. In those with severe radiographic OA of the knee, no significant symptomatic benefit could be demonstrated. The study did not evaluate patients for structure or disease modification.
In the Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT),115 1583 patients with OA of the knee were randomized to placebo, glucosamine hydrochloride 1500 mg/day, chondroitin sulfate 1200 mg/day, celecoxib 200 mg/day, or glucosamine hydrochloride and chondroitin sulfate. The primary endpoint was the percentage of patients achieving at least 20% improvement on the WOMAC pain subscale at 6 months. The only statistically significant response was seen in those on celecoxib versus placebo (70.1% vs. 60.1%; P =.008). Patients were then stratified for baseline severity by WOMAC pain scores, most of them falling into the mild OA pain category. In a subgroup analysis, in those with moderate to severe OA pain (WOMAC pain, 301 to 400 mm), the combination of glucosamine hydrochloride and chondroitin sulfate was more efficacious than placebo as measured by a dichotomous response rate (positive = 50% improvement in pain): 79.2% versus 54.3% (P =.002). Analysis of the radiographic data from this trial failed to support the notion of a disease-modifying/slowing role for glucosamine, chondroitin sulfate, or the combination of these compounds.
From these results, it appears that patient selection may be important in maximizing any potential benefit from glucosamine or chondroitin therapy. The GAIT study also had a particularly high placebo response rate, which may reflect the enrollment of patients with less symptomatic OA and may have affected the results. It also used a glucosamine hydrochloride preparation instead of the glucosamine sulfate used in most other studies, particularly those that have demonstrated efficacy. This raises the question of whether the choice of glucosamine hydrochloride negatively affected efficacy in the trial. However, one small (142 patients) Chinese trial randomized patients with OA of the knee to glucosamine sulfate 1500 mg/day or glucosamine hydrochloride 1440 mg/day for 1 month.116 No efficacy differences were noted, with a clear majority of patients in each treatment arm achieving symptomatic improvement by Lequesne scores. The study had no placebo arm. Safety assessments continued for 2 additional weeks, with no significant adverse events reported. At present, it is still unclear whether glucosamine hydrochloride preparations have the same potential clinical benefits as glucosamine sulfate preparations. Additional investigations are necessary.
Two European trials tried to address the subject of disease modification with glucosamine. In one study, 212 patients with OA of the knee were randomized to receive placebo or glucosamine sulfate (1500 mg/day) and were followed prospectively for 3 years.117 Fluoroscopically positioned, standing anteroposterior radiographs of the knees were taken at enrollment, 1 year, and 3 years. At 3 years, the treatment group had a joint space reduction of 0.06 mm, whereas the placebo group had a reduction of 0.31 mm. Whether this is a clinically meaningful difference in joint space is unclear. Those taking glucosamine also showed symptomatic benefit on the order of 20% to 25%, whereas those taking placebo had a slight worsening of symptoms, as judged by the WOMAC. There were no significant adverse events attributed to the use of the glucosamine sulfate. A second group of researchers randomized 202 patients to receive placebo or glucosamine sulfate (1500 mg/day) for 3 years.118 The width of the narrowest medial joint space of the tibiofemoral joint was measured serially, using visual assessments with a 0.1-mm graduated magnifying glass on standardized full-extension, weight-bearing anteroposterior radiographs of each knee. At 3 years, there was a significant difference in joint space width, with a decrease of 0.19 mm in the placebo group and an increase of 0.04 mm in the glucosamine sulfate group. Also, significantly greater improvements in the WOMAC score and the Lequesne index were seen in the glucosamine group. The favorable results of these studies have been questioned because of the radiographic technique used to assess joint space. At issue is whether the joint space seen on standing films of the knee might be significantly affected by the symptoms of OA (i.e., pain) and whether a semiflexed film would be preferable. In one study, investigators obtained baseline radiographs (after analgesic or NSAID washout) using both standing-extended and semiflexed, fluoroscopically positioned techniques in 19 patients with knee OA.119 Radiographs were then repeated 2 to 8 weeks later after reinstitution of analgesic or NSAID therapy. Joint space width increased with effective pain relief in highly symptomatic patients if measured by standing-extended radiographs. Using the semiflexed technique, there were no significant changes in joint space width related to severity of pain or responsiveness to pain therapy. This suggests that data obtained using the standing-extended radiographic technique may need to be revisited because the results may represent a therapeutic intervention’s effect on symptoms (pain) rather than a disease-modifying effect. More recent, ongoing trials have changed to the semiflexed, fluoroscopically positioned knee radiograph to assess potential disease modification.120
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree