CHAPTER 10 The shin
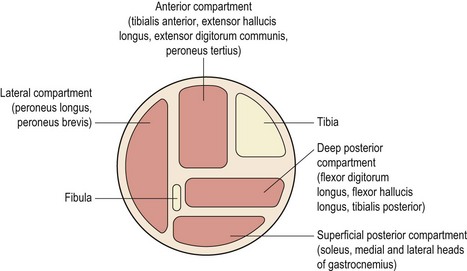
The term ‘shin splints’ is often used as a blanket description of any persistent pain occurring between the knee and ankle in an athlete. Originally called ‘fresher’s leg’, a more accurate description of this type of exercise induced leg pain comes with the various ‘compartment syndromes’ which identify the anatomical structures affected. The anterior compartment contains the tibialis anterior, extensor hallucis longus, extensor digitorum longus, and the anterior tibial artery and vein. The lateral compartment contains the peronei and the superficial peroneal nerve. The superficial posterior compartment contains the gastrocnemius and soleus and the deep posterior compartment contains the tibialis posterior, flexor digitorum longus, flexor hallucis longus, the peroneal artery and vein, and the posterior tibial artery and vein (Fig. 10.1). The deep and superficial posterior compartments are separated by the deep transverse fascia of the leg.
Compartment syndrome is an exercise induced leg pain which can affect several structures (Table 10.1). Direct bone pain may occur with a stress fracture or stress reaction, and indirect bone pain can come from traction through muscle fascia causing inflammation of the periosteum. Periosteal inflammation (periosteal contusion) may also occur through direct trauma such as a kick to the shin. Muscular pain may be acute immediately following exertion, or chronic building over time. Chronic pain may eventually become noticeable even at rest. Neural compression may be local or pain referred from nerve root compression in the lumbar sacral spine. Finally, vascular signs may exist in cases of popliteal artery entrapment.
Table 10.1 Structures affected in compartment syndrome
MTSS – medial tibial stress syndrome.
After Padhiar (2009).
Anterior compartment syndrome
Anterior compartment syndrome involves pain in the anterior lower leg (Fig. 10.2A), which is increased in resisted dorsiflexion. There is usually a history of a sudden increase in training intensity, frequently involving jumping or running on a hard surface. The anterior compartment muscles swell, and in some cases hypertrophy occurs. The fascia covering the muscles may be too tight and inflexible to accommodate the increase in size. As a consequence, when the muscles relax, their intramuscular pressure remains high and fresh blood is unable to perfuse the tissues freely. This decrease in blood flow leads to ischaemia with associated pain and impairment of muscle function.
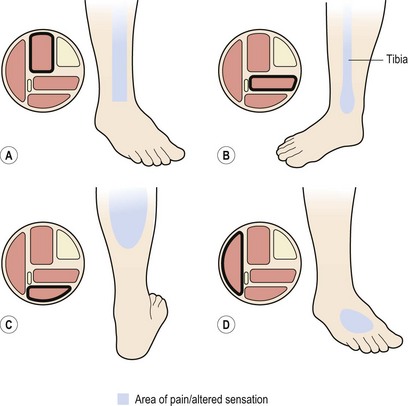
Figure 10.2 (A) Anterior compartment syndrome. Pain on the anterolateral aspect of the lower leg. (B) Deep posterior compartment syndrome (medial tibial stress syndrome). Pain over distal third of tibia (see also Fig. 10.3). (C) Superficial posterior compartment syndrome. Pain within the calf bulk. (D) Lateral compartment syndrome. Loss of sensation over the dorsum of the foot.
Usually, when a muscle contracts, its blood flow is temporarily stopped. Arterial inflow occurs once more between the muscle contractions as the intramuscular pressure falls. Normal resting pressure within the tibialis anterior in the supine subject is about 5–10 mmHg, increasing to as much as 150–250 mmHg with muscle contraction. Muscle relaxation pressure, that which occurs between repeated contractions, is between 15 and 25 mmHg in the normal subject, but in athletes with anterior compartment syndrome pressures may rise to 30–35 mmHg and take up to 15 minutes to return to normal values (Styf, 1989).
The intracompartment pressure (ICP) may be measured using a catheter introduced into the painful compartment via a cutting needle (medicut or similar). Saline and heparin are flushed into the area and the catheter is linked to a pressure transducer. A non-invasive technique has been developed which uses a 5 mm diameter indenter to measure quantitative hardness of the shin compartment to objectively measure the tissue tension which clinicians routinely measure subjectively. The use of this non-invasive compartment syndrome evaluator (NCSE) correlates well (Pearson correlation coefficient of 0.84) with direct measurement using a catheter (Steinberg, 2005).
The exact cause of the condition is not known. Hypertrophy may be one factor, as the condition occurs frequently when training intensity is increased. However, bodybuilders rarely suffer from the condition, so the rate rather than the amount of muscle hypertrophy may be important. Microtrauma and excessive stress to the blood capillaries and lymphatics may give rise to inflammation and in some cases myositis in the area (Styf, 1989).
Lateral compartment and superficial peroneal nerve
This is an unusual cause of shin pain and occurs when the peroneal muscles are affected, usually by hyperpronation (Brody, 1980). The condition may have existed for some time but is brought to the fore when running begins. Again, there is ischaemia and pain, but in addition the superficial peroneal nerve may be compressed as it emerges from the lateral compartment.
The nerve lies deep to the peroneus longus and then passes forwards and downwards between the peronei and the extensor digitorum longus. It pierces the fascia in the distal third of the leg where it divides into medial and lateral branches to enter the foot. Entrapment may occur if muscle herniation or fascial defect exist. In addition, ankle sprain, fasciotomy and an anomalous course of the nerve have been suggested as contributory factors (Styf, 1989). Clinically, the patient presents with loss of sensation over the dorsum of the foot, especially the second to fourth toes (Fig. 10.2D). Certain resting positions may compress the nerve and bring on the symptoms. To test the nerve, it is compressed over the anterior intermuscular septum 8–15 cm proximal to the lateral malleolus while the patient actively dorsiflexes and everts the foot. Tinel’s sign, involving local percussion over the compression site, may be positive.
Posterior compartment
The superficial compartment contains the soleus and gastrocnemius. These muscles are usually affected by trauma rather than ischaemia, and are dealt with separately below. Pain occurs within the calf bulk (Fig. 10.2C) and is increased with resisted plantarflexion.
The deep posterior compartment contains tibialis posterior, flexor digitorum longus and flexor hallucis longus, and is the most common site for shin pain. Pain in this region is usually experienced over the distal third of the medial tibia (Fig. 10.2B) and represents medial tibial stress syndrome (MTSS; Mubarak et al., 1982). The exact site of pain will vary depending on the specific structures affected, and Detmer (1986) described a system of classification involving three types of chronic condition (Fig. 10.3).
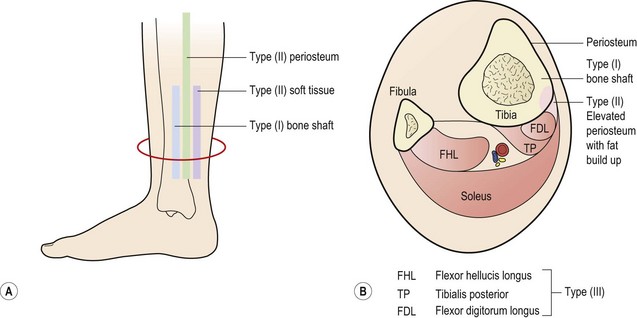
Figure 10.3 Medial tibial stress syndrome. (A) Painful areas of palpation. (B) Cross-section of structures affected.
After Detmer, D.E. (1986) Chronic shin splints: classification and management of medial tibial stress syndrome. Sports Medicine, 3, 436–444. With permission.
Type II medial tibial stress syndrome involves the junction of the periosteum and fascia, and occurs particularly in sprinters and those involved in jumping activities. Pain is maximal just posterior to the bone, and has often persisted for a number of years. Initially, pain occurs only with activity, but as the condition progresses discomfort is felt with walking and even at rest. In this condition compartment pressures may not be elevated, and the periosteum is unchanged. One explanation is that the periosteum is traumatically avulsed from the bone by the action of soleus through its attachment to the fascia. During the chronic stage of this condition, adipose tissue has been found, during surgery, between the periosteum and underlying bone (Detmer, 1986). In the early stages of the condition the periosteum may heal back with rest, but when the condition becomes chronic, it is unable to heal, and continues to cause pain when stressed by activity.
Management
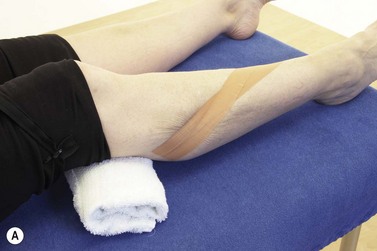
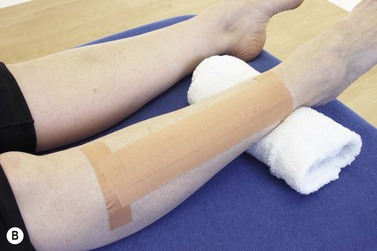
Initial management of shin pain involves a reduction of the stresses which caused the condition in the first place. This involves accurately identifying the structures affected and taking a thorough history of causal factors, particularly stresses imposed during training. Temporary pain relief may be achieved using deload taping to limit the fascial traction thought to be part of the pathology. Taping is applied in a spiral beginning on the lateral aspect of the ankle just above the lateral malleolus. The tape is wound around the shin going behind the calf and emerging once more onto the front of the tibia. As the tape is applied it is pulled proximally to unload the fascia (Fig. 10.4A). Another approach is to place anchors just below the knee and just above the ankle, and connect the anchors with 2 or 3 strips of zinc oxide taping gathering the skin up to take tension away (Fig. 10.4B). Both tapes alter the fascial loading and give temporary relief only.
Biomechanical assessment of the lower limb is mandatory and prescription of orthotics should be made where necessary. Initially, rest and anti-inflammatory modalities are used to allow the acute inflammation to settle, but external compression and elevation of the limb may exacerbate the problem (Di Manna and Buck, 1990). If training stresses can be modified, and the condition has been identified early enough, this may be all that is required.
Treatment note 10.1 Manual therapy for shin pain
Trigger point massage
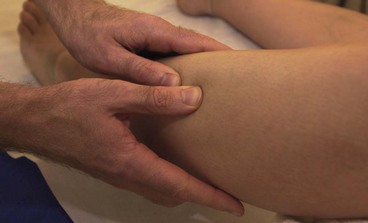
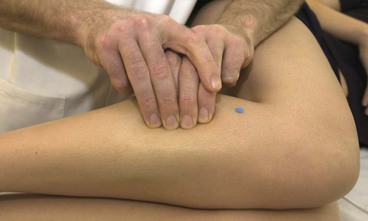
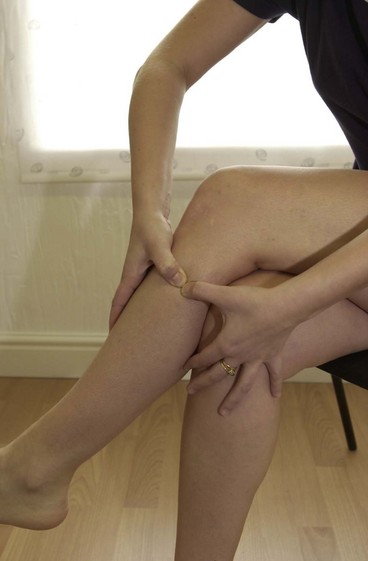
Trigger point (TrP) massage for anterior compartment syndrome focuses on the tibialis anterior and the extensor digitorum longus. The TrP for the tibialis anterior is located approximately one-third of the way distally from the knee and to the lateral side of the tibia. A muscle stripping technique can be used starting from half-way down the tibia progressing up towards the knee in a slow movement gradually progressing in depth (Fig. 10.5). The thickness of the muscle means that both thumbs must be used simultaneously or a pressure tool where ischaemic compression is used. The elbow is the tool of choice, direct compression is given and maintained for 3–10 seconds or until pain subsides.
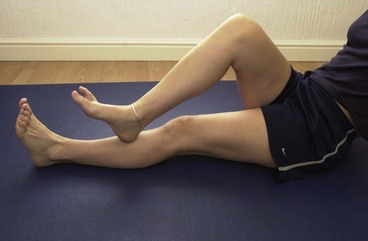
The long toe extensors (extensor digitorum longus (EDL) and extensor hallucis longus (EHL)) may be similarly treated. The EDL TrP is located approximately 8 cm distal and slightly anterior to the head of the fibula. The EHL is located at the junction of the middle of the distal thirds of the lower leg (Fig. 10.6). Home stretching may again be used, this time forcing the foot into plantarflexion and the distal toes into flexion.
Ischaemic compression may be used with the thumbs and ice stretch may again be given, this time stretching the foot into plantarflexion and inversion (Fig. 10.7).
Posterior compartment syndrome and medial tibial stress syndrome require treatment of the flexor digitorum longus (FDL) and tibialis posterior along the lower third of the posterior edge of the tibia. Where the muscle itself is stressed, the TrP is targeted on the medial border of the upper tibia. The patient lies in crook side lying and the palpation point is between the medial edge of the tibia and the gastrocnemius muscles (Fig. 10.8). The gastrocnemius is pushed posteriorly and the pressure is then applied downward and then laterally. For the flexor hallucis longus (FHL), the patient may be treated in prone and this time the thumb is positioned lateral to the mid-line, pressing on the edge of the soleus at the junction between the middle and lower thirds of the lower leg.
Self-help trigger point methods
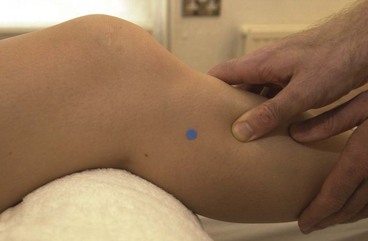
The athlete may be taught to use trigger point therapy as a self-treatment to relieve pain as it occurs. For the tibialis anterior, the easiest method is to use the heel of the opposite foot (Fig. 10.9). The movement is on the lateral edge of the tibia and begins at mid-shin level, the pressure being in a continuous sweep towards the lateral edge of the knee.
For the peroneal muscles, pressure may be given by the index finger, supported by the middle finger or by a single flexed knuckle (Fig. 10.10). For the flexor digitorum longus and tibialis posterior, one or two fingers may suffice. Alternatively, hands may wrap around the shin and both thumbs may be used to apply to the peroneus longus.
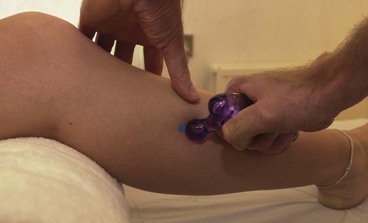
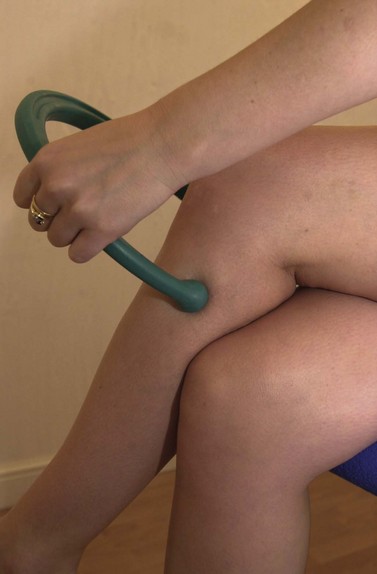
Where pressure on the trigger points is too tiring for the hands, apparatus such as the ‘backnobber’ (Physiomed, Manchester, UK) can be used to create more force (Fig. 10.11).
Stress fractures
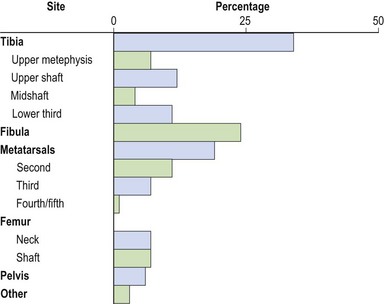
Over 50% of all stress fractures occur to the tibia and fibula (McBryde, 1985) with the remaining sites being mostly to the lower limb (Fig. 10.13). Stress fractures are usually the end point in a sequence of overuse. A number of causal factors usually coexist to begin the development of the condition. Training errors may account for 60–75% of such injuries in runners (McBryde, 1985). Common faults include high intensity work carried out for too long with an inadequate recovery, for example a distance runner who suddenly increases mileage. Faulty footwear which fails to attenuate shock and exercising on unforgiving surfaces will also contribute to lower limb pathology. These factors, coupled with an underlying malalignment problem of the lower limb (or biomechanical faults in technique of upper limb actions, see Chapter 5), will exacerbate the problem. With novice runners, an additional factor is muscular weakness in the lower extremity leading to a reduction in shock-absorbing capacity of the soft tissues. In each case, the overload on the tissues exceeds the elastic limit, causing a plastic deformation of bone.
Very often, the stress fracture is a direct result of a change of some type – in the athletes themselves, in the environment in which they train, or in the activity (Garrick and Webb, 1990). In terms of the athlete, stress fractures often emerge following the onset of a growth spurt or at the menopause as a result of the large body adaptations occurring at these times. Similarly, following illness, the body must be allowed time to readapt to training demands. Environmental changes, such as new clothing (shoes) or a new playing surface, will also require time to allow tissue adaptation, and failure to allow for this may lead to tissue breakdown, of which stress fractures are one type. Finally, alterations in the quality or quantity of a training programme itself will require a period of adaptation.
Examining excessive running and jumping in rabbits, Li et al. (1985) demonstrated that osteoblastic activity occurred from 7 to 9 days later than osteoclastic activity. Remodelling began on day 2, with the haversian blood vessels dilating, and by day 7 osteoclastic activity was noted in the bone cortex. New bone formation began in the periosteum by the 14th day of excessive stress. The adaptation to this excessive stress was reabsorption which occurred for some time before the formation of new cortical bone, thus weakening the bone structure. Abnormal x-rays were not found until day 21 after the stress was imposed.
Signs and symptoms
The main symptom of a stress fracture is pain. This has been categorized into four types, depending on its characteristic (Puffer and Zachazewski, 1988). With type I pain, the athlete only feels discomfort after activity. With type II and III pain, discomfort is felt while training, but with type II this does not restrict activity. Type IV pain is chronic and unremitting in nature. Further symptoms include warmth and tenderness over the injured area, made worse by sporting activity and better with rest. Swelling may be evident in the later stages of the condition if the bony surface is superficial. Accuracy of palpation when assessing bone pain is vital. The tenderness of a stress fracture is usually well localized, whereas that of compartment syndrome is more diffuse. Initially radiographs are usually negative, it taking at least 2 weeks for x-ray changes to be apparent (Li et al., 1985; Rzonca and Baylis, 1988). Local periosteal reaction and new bone growth may be seen after 6 weeks in long bone (Markey, 1987) and compressive stress fractures in cancellous bone are sometimes visible after 24 hours (Puddu et al., 1994). Taunton and Clement (1981) found radiographs to be positive in only 47.2% of their cases, while bone scan was accurate in 95.8%. Bone scan is normally revealing at the onset of symptoms and is generally more reliable. Phosphate labelled with technetium-99 m is incorporated into osteoblasts and a hot spot appears over the active area, 6–72 hours after the onset of pain (Puddu et al., 1994).
Pain may be produced over the superficial fracture site by vibration. This may be produced from a tuning fork or ultrasound unit (Lowden, 1986), and is generally of more use in low risk areas, such as the foot and shin. Where there is a risk of complication through displacement (such as in the neck of the femur) bone scan with possible surgical intervention may be more appropriate (Fig. 10.14).
Treatment
Treatment of a stress fracture is primarily that of rest. As a general guide, with type I or II pain the workload should be reduced by 25% and 50%, respectively. Total rest is called for where type III pain is experienced, and type IV pain requires immediate medical investigation (Table 10.2). In the more severe conditions, rest should be total, because even allowing an athlete to train the upper body will often result in ‘just trying the leg out’ in the gym. Training should not resume until the athlete has been totally pain free for 10 days. It should be emphasized to the athlete that this means at the end of each day he or she should go to bed having felt no pain over the injured area during that day.
Table 10.2 Pain classification
| Classification | Characteristics | Action |
|---|---|---|
| Type I | Pain only after activity | ↓ workload by 25% |
| Type II | Pain during activity but not restricting performance | ↓ workload by 50% |
| Type III | Pain during activity restricting performance | Total rest |
| Type IV | Chronic unremitting pain at rest | Splint/cast Medical investigation |
After Puffer and Zachazewshi (1988).
The timescale of healing will vary depending on the site of injury. With reduced activity, fibular stress fractures will normally heal in 4–6 weeks, tibial stress fractures in 8–10 weeks and femoral neck stress fractures in 12–16 weeks (Reid, 1992).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree