The management of osteomyelitis
INTRODUCTION
Medical care of the elderly will assume a greater proportion of most physicians’ practice in the future and thus orthopaedists must have a better understanding of how to manage the unique ailments of the geriatric patient. Infection will continue to be a significant complication of all invasive surgical specialties. Unique to the geriatric population are both systemic and local physiologic changes that make care of geriatric infections much more challenging. Using the Cierny–Mader classification of osteomyelitis, the patient is characterized by both infection status and host status (Figures 5.1 and 5.2). The host status is reflective of systemic and local extremity influences that exist due to a variety of factors. The geriatric patient is almost always by definition a B-systemic and B-local host because of changes in the vascular and immunologic systems. Even though geriatric patients demonstrate healing capacity, their existing issues and postoperative demands and restrictions make the care of geriatric infections challenging.
Bacteria are an essential part of human existence with trillions of bacteria living within and on our bodies at any given moment. This normal flora represents a symbiotic relationship between the host and microbacterial world and demonstrates that the simple existence of bacteria does not equate to an infectious process. In fact, most bacterial–host interactions are beneficial for both entities and it is only when there is some imbalance in the relationship or introduction of a new or altered state that a potential problem may arise. Bacteria are needed for proper digestion as well as defence against certain more virulent strains. The host bacteria are often competing among themselves in this symbiotic relationship. Thus, a positive culture, which can easily be obtained from nearly any body part, does not necessarily equate to an infection or require treatment. A study conducted by Moussa et al. highlighted this phenomenon and found that nearly 50% of cultures taken during hardware removals were positive in patients who had no evidence or symptomatology of infection.1 Our understanding of infection and the relationship between humans and bacteria continues to evolve, especially the geriatric population who continue to increase their life expectancy. This chapter addresses osteomyelitis in the geriatric population highlighting the various clinical, laboratory and imaging diagnostic methods and the multidisciplinary treatment approach needed to treat this difficult problem.
PATHOPHYSIOLOGY
Infections in orthopaedics typically result from some type of trauma or may have an iatrogenic cause after internal fixation or arthroplasty. Bacterial colonization is a necessary first step but may not be sufficient to cause infection. Colonization is typical of biofilm-forming bacteria and this section reviews the pathophysiologic processes involving the normal host defense mechanisms, the formation of colonies and the evolution from colonization to infection as well as the host response to infection.
Normal host defenses
The host immune system is constantly fighting against breaches that may threaten host existence. It is estimated that at any given moment there are millions of bacterial invasions being neutralized by host defense mechanisms. For example, there are over 180 different types of bacteria on our skin and over 400 bacterial species in one section of large intestine at any given moment.2,3 These host defense mechanisms are complex and outside the scope of this chapter. However in summary the host uses B cell defenses primarily against bacteria, T cell defenses primarily against viral invasions and the macrophage system against foreign bodies.4 Healthy hosts are able to defend against most bacterial species that enter and exist in isolation (called the planktonic state and intended to be analogous to plankton floating in the ocean). As long as bacteria are in the planktonic state, they are susceptible to host defenses, which attack and neutralize the bacterial invasion through phagocytosis, oxidative enzymatic destruction and a number of other mechanisms. If the bacteria evade the host defenses, they must then find a suitable surface or location to begin adherence and initiate their reproductive cycle and form colonies which ensures their ongoing survival. Bacterial adhesion is the first step but can only occur on an inert surface, such as a foreign body, or on non-viable or necrotic tissue. Implants and traumatized tissues are very suitable surfaces that can harbor bacteria and allow colonization by virtue of being outside the normal vascular or living intercellular spaces. They are also remote from any antibiotic agent that requires access to bacteria for efficacy. The presence of a foreign body decreases the minimal abscess-forming dose of Staphylococcus aureus by at least 10,000-fold in the animal model as well as in humans.2 Numerous phenomena and interactions are involved with bacterial adhesion but the formation of a colony usually requires bacteria that have evaded host defenses long enough to begin a geometric reproductive cycle. Reproductive cycles of bacteria are geometric and have been reported to occur every 20 minutes and increase in an exponential manner.5
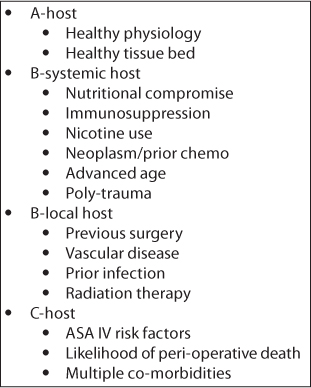
Figure 5.1 Cierny–Mader classification of osteomyelitis showing the types of hosts.
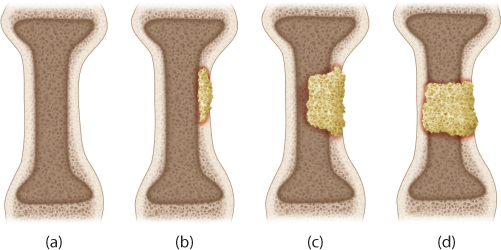
Figure 5.2 Cierny–Mader classification of osteomyelitis showing the types of bony lesions. (a) Medullary osteomyelitis. (b) Superficial osteomyelitis. (c) Invasive osteomyelitis with axial stability maintained. (d) Invasive osteomyelitis with loss of axial stability.
Host defenses will identify and attack bacteria and begin a systematic process of destruction (oxidative and enzymatic processes) as well as recruitment of other host cells (chemotaxis) to aid in the destruction of any invasion. As in wars of attrition, an imbalance that results in either a mitigated host response (immune suppression) or an overwhelming bacterial invasion (inoculation) may tip the balance in favour of bacterial species and allow reproduction and colony formation. Immunologic function in humans varies from birth to death and vulnerabilities exist in various ages. In the elderly, there is a known reduction in the immune response which will predispose the elderly to such imbalances between bacterial invasion and defense mechanisms. The immunologic changes in the elderly include a decline in the normal lymphoid cell activity known as immunosenescence. The hematopoietic stem cells diminish in capacity which in turn leads to a decrease in cell lines including phagocytes and lymphocytes.6 This decline in the lymphocytic cell line affects both function and production. In addition to changes in immune responses, the usual beneficial effects of inflammation present earlier in life become detrimental to the elderly host. It should be further noted that changes in the lymphoid cell line are not solely responsible for the malfunctioning of the immune system in the elderly. Even though the myeloid cell line does not diminish with normal aging, macrophages can become improperly regulated as a consequence of environmental changes.7 All of these changes in the elderly lead to a decreased immune response and a more advantageous environment for bacterial colonization and subsequent infection.
Adherence and colonization
Postsurgical or traumatized tissues are perfect environments for bacterial adherence and the initiation of colony formation. However, colony formation is not guaranteed because of one very important host defense mechanism, tissue integration. Concomitant with host defense systems fighting against bacterial invasion is the host response to the inert surface of dead tissue or an implant. Host cells will attempt to eradicate dead tissue through oxidative processes and replace the dead tissue with intact, living host tissue. Implants that are biocompatible will often be covered with a neo-membrane of tissue integration that is not inert and therefore resistant to bacterial adhesion. This is the first step. Vascular tissue can also provide a conduit for the delivery of antibiotics and more host immune cells and thus reduce the chances of colonization. Bacteria that are trapped or ‘sequestered’ are either slowly killed or become quiescent through adaptive metabolic changes. Such a phenomenon would explain the previous findings of Moussa et al. which showed that asymptomatic hardware retrievals resulted in culture positive specimens.1 Thus the ‘race for the surface’ is the inciting event that will often determine whether colonization, and potential infection, or bio-integration occurs. The time period between injury and implantation may allow useful interventions to help favor the host versus bacterial infection.
Colony formation begins a complex process and bacteria can metastasize and form new colony forming units. Colonies have been found to have some ability to ‘communicate’ with primitive signaling mechanisms and develop their own defenses against the host response. Once colonization occurs, bacteria can begin to form a mucopolysaccharide glycocalyx protective film that is colloquially called ‘slime’. This protective layer serves multiple purposes and helps maintain the colony while also protecting it against host defenses. The glycocalyx membrane will prevent penetration of host cells and their oxidative enzymes as well as serving as a diffusion barrier against antibiotics. This nearly impermeable matrix is so strong that antibiotic concentrations upwards of 1000 times normal may be required to just reach the bacteria encapsulated in the colony biofilm.8 Even if antibiotics reach the bacteria, their main mechanism of function is either cell wall disruption or interference with the nuclease function required for reproduction.2 One of the features of mature colonies is the lower metabolic state of encapsulated bacteria. The lower metabolic state may be thought of as a sort of hibernation that results in the appearance of so-called ‘pseudo-resistance’. If bacteria are not actively reproducing or metabolically active, any effect on the cell wall such as transport of nutrients or nuclease production will not be realized and while the bacterial species may still be sensitive to the antibiotic in their active state, their lack of active reproduction will appear as resistance and allow antibiotic concentrations to lapse. Thus, in a short period of time after the cessation of antibiotic treatment, the antibiotic will diffuse away, and if the bacteria emerge into a more metabolically active state, the antibiotic will be perceived to have failed. This phenomenon helps explain the waxing and waning characteristic of chronic infections and osteomyelitis.
Another effect of biofilm interfering with the host response is auto-injury. As host immune cells react to local signaling proteins and begin to recruit other cells and begin their ‘attack’ on the encapsulated cells, the release of their oxidative enzyme can potentially result in harm to intact local host tissue. The accumulation of white cells and local tissue damage typically manifests as the purulence and inflammation of infection. While some bacteria also contain endotoxin and harmful agents that may be damaging in their effect, such as the toxins of necrotizing fasciitis, much of the local tissue damage in an active infection may actually be the result of the host attempting to eradicate encapsulated organisms. As the condition becomes more chronic, the host will ‘sequester’ the effort to localize it and prevent systemic spread. Sequestra and sinus tracts are the results of this effort and characterize chronic infectious states. However, despite host efforts to isolate infectious processes in the elderly, systemic spread may be difficult to prevent due to the compromised local and systemic state of the host.
CLASSIFICATION
The numerous osteomyelitis classification systems use time, aetiology, mechanism or management as the basis for classification. Osteomyelitis has been described as either acute or chronic based on the length or duration of symptoms. Acute osteomyelitis can be characterized as an infection presenting with oedema, small vessel thrombosis and vascular congestion within 2 weeks of onset. Chronic osteomyelitis is defined as osteomyelitis lasting more than 6 weeks or a recurring infection after appropriate treatment.9 This type of osteomyelitis often occurs in conjunction with conditions of vascular compromise such as peripheral vascular disease or diabetes mellitus. It is not uncommon for both of these comorbidities to be present in an elderly patient with osteomyelitis. While the temporal definitions appear rather arbitrary, and not based on any true science, the intent is to help differentiate infectious processes that might require different treatment paradigms. Acute infections may potentially be eradicated with less invasive or regressive methods, whereas a more mature infection requires more aggressive or staged techniques.
Aetiologic classifications of osteomyelitis focus on the mechanism of occurrence, such as route of entry. Hematogenous causes are a result of a bacteremia from whatever cause. Oral procedures, trauma or surgical procedures can introduce an inoculum of bacteria into the blood stream. Any bacteria escaping host defense efforts may manifest themselves in another part of the body. This process is one of the main reasons empiric or prophylactic antibiotic treatment was advocated for patients with prostheses undergoing invasive dental procedures. While not absolutely proven or disproven, the known bacteremia from oral procedures and the occurrence of infection after such a procedure in the absence of other explanations remains an empiric, or non-evidence based, explanation for its occurrence and treatment. Iatrogenic and nosocomial infections are frequently caused by species of Staphylococcus, Streptococcus or enteric organisms. As it is commonly perceived that hospital acquired infection (HAI) and surgical site infection (SSI) are avoidable, there are numerous regulatory and financial efforts to mitigate their occurrence. In reality, the incidence of such infections will probably never be zero and some low, although acceptable, incidence of these infections should probably become the normative benchmark. Until regulatory agencies acquire such a reasonable understanding of infection, the efforts put forth towards mitigating such infections are well intended but potentially confusing to both provider and patient.
The most common classification of osteomyelitis is that of Cierny and Mader which focuses on describing bone pathology and the status of the host or patient (Figures 5.1 and 5.2). In this system there are four main considerations when classifying osteomyelitis: the host condition, the functional impairment caused by the insult, the involved site and the extent of bony necrosis. The most compelling finding of the Cierny–Mader study was that probably the most important factor for outcome was the physiologic status of the host.10
In the Cierny–Mader system, the ‘type A’ host has normal immune status, with healthy local and systemic physiology (Figure 5.1). The ‘type B’ host is immunocompromised to some degree and is further subclassified into B-systemic and/or B-local compromised groups. Examples of B-systemic compromise include vascular disease, malignancy, diabetes, poor nutritional status, renal or hepatic insufficiency, nicotine or substance abuse and immunocompromising diseases or treatments. Examples of B-local compromise include local or previous limb cellulitis, lymphedema, previous surgery, radiation treatment, peripheral vascular disease and any trauma including minor or major injury. The C host is a specific type of host that is compromised to such an extent that Cierny described it as someone where ‘treatment to cure the infection was worse than the infection itself’. In these patients, chronic suppression, amputation or death is often the only choice. Taking this classification into consideration, the geriatric population will likely provide very few A hosts, and most will start as B-systemic and/or B-local hosts presenting as ‘type C’ hosts with few curative options available.
The Cierny–Mader system also describes the bone lesion as having four distinct types (Figure 5.2). Type I osteomyelitis is a medullary osteomyelitis with an endosteal nidus. The bone is axially stable and the cortex is infrequently permeated. Its cause has been proposed to be haematogenous spread or seeding. It is typically seen in children and adolescents where a bacteremia will seed the injured area of the hypovascular region of the growth plate. This lesion does not typically require any form of bone grafting procedure and many can be treated with either local treatment or antibiotics alone. Type II osteomyelitis is a superficial osteomyelitis that affects the outer surface of the bone. It does not permeate the cortex and is axially stable. It is typical of pressure ulcers or outside-in inoculations. Local treatment and soft tissue coverage will usually provide definitive cure. Type III osteomyelitis involves both cortical and medullary bone but is by definition an infection that maintains axial stability or continuity of the bone. This type of osteomyelitis is typically labeled after excision and may often be due to what appears as a type II lesion becoming a type III lesion after treatment. The axial stability is not necessarily sufficient to allow weight bearing, but it does not require osseous reconstruction to result in an axially stable limb that can eventually become weight bearing. Type IV osteomyelitis is a permeative destructive lesion that causes instability because of segmental cortical and medullary involvement. This lesion results in osseous discontinuity that will require some type of reconstruction either initially or in a staged manner. Frequently, what appears or presents as a type III lesion will become a type IV lesion after appropriate treatment. Both type III and type IV lesions are commonly seen after fracture or traumatic injuries (Figure 5.1).
One of the main benefits of this classification system is the data and outcomes provided by Cierny and Mader. They found that A hosts achieved up to a 98% success rate even with type III and type IV lesions. B hosts had success rates ranging from 79% to 92% and in such patients preparatory interventions such as improving disease states or nutrition had the greatest impact.11 The take-away message from their work is that the host’s physiologic condition plays just as integral a role, if not more, as proper treatment of the osteomyelitis. The physiologic importance of the host is magnified in the elderly population and the effect of physiologic optimization in geriatric patients with osteomyelitis cannot be overstated. In the elderly, systemic and prior local compromise is almost always present. Even in the very healthy elderly there is undoubtedly some systemic compromise in cardiovascular and immune function that must be taken into account. The most compelling intervention attributed to Cierny’s work is improvement of the host status. We know this to be true when the elderly present with a fractured hip. They are frequently dehydrated with electrolyte abnormalities, and they often have diabetes, cardiac disease, neurocognitive dysfunction and poor physiologic reserve. Such patients are never really ‘cleared for surgery’. Instead their physiologic status is optimized for the necessary surgical intervention. With infection the same process should occur as undertaken for hip fractures, but because the infection may not be as acute a condition there is opportunity to improve host status even further. Optimizing nutrition, normalizing serum protein, improving tissue oxygenation and withholding some cytotoxic agents are all relatively easy to achieve even in the elderly. Even though this is also done in the non-elderly population, the geriatric host provides an excellent opportunity to optimize a host that may benefit the most from preoperative intervention.
DIAGNOSIS
The diagnosis of osteomyelitis in the geriatric population can be made using existing clinical and laboratory tools in conjunction with imaging modalities. Symptoms can be vague and normal signs of overt infection can be absent due to a blunted host response. Patients may complain of generalized fatigue, malaise, fever and lethargy. Local signs like swelling, warmth, erythema and drainage may or may not be present in the affected area. A high index of suspicion should be present when an elderly patient has a history of infection at another site, such as cellulitis, pulmonary infection or urinary infection, in combination with an orthopaedic injury or disease. Specifically genito-urinary problems occur in the elderly that can result in transient bacteremia or the spread of infection through Batson’s plexus into the spine. The literature supports an association between existing urinary tract infections and prosthetic infection.12 The elderly may also have poor dentition that can result in infection. Patients with a history of prior trauma or who are immunocompromised should prompt the clinician to undertake further investigations.
The most common screening tests are the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and complete blood count (CBC) to look for other potential conditions, such as hematologic neoplastic disorders, that mimic infection. Blood cultures are unlikely to be helpful unless systemic symptoms are present but they are usually undertaken in any workup for osteomyelitis. Elevations in the white blood cell (WBC) count, ESR and CRP are suggestive of an infection but remain non-specific markers of inflammation. When the CRP and ESR are combined, the specificity increases to 90–95%. If both are negative, it is unlikely that acute infection is present.13 Greidanus et al. looked at ESR and CRP in assessing infected arthroplasties and found that these parameters were poor for screening but had high specificities and negative predictive values, which were helpful in making treatment decisions.14 It is important to understand that the measurement of ESR and CRP is only one tool that clinicians should use as a part of their armamentarium for diagnosing osteomyelitis in the geriatric population. These values can and will be elevated for up to 6 months in a patient who has had recent surgery and they may be normal in a patient with a chronic infection. Additionally, both ESR and CRP are helpful once a diagnosis of osteomyelitis has been made since they provide a baseline value to monitor before, during and after treatment.
Once the decision has been made to proceed with operative treatment, biopsy and culture should be performed to try to identify the infective organism. Superficial cultures are not helpful and can even lead to inappropriate antibiotic selection and promotion of antibiotic resistance. Deep cultures and bone biopsy are the preferred diagnostic tools for osteomyelitis. Zuluaga et al. found that non-bone specimens produced 52% false negatives and 36% false positives.15 This is not to say that superficial and non-bone specimens cannot be helpful. However, they should be evaluated critically relative to multiple bony specimens in order to maximize the potential for correct diagnosis. Even when bony specimens are obtained, no organism is recovered in up to 50% of cases. The low recovery and identification is most likely due to sampling error or partial treatment. Therefore, numerous cultures and specimens are recommended. Staphylococcus aureus remains the most common infecting agent that is recovered and represents 80–90% of all infections.16 The clinician must also be aware that other potential pathogens exist concomitantly with the more common species. For instance, Gram-negative rods can occur in the same area and the same infection as the expected strains of S. aureus. Many bacteria can co-exist in a wound and in osteomyelitis, but polymicrobial infections without a dominant organism may be especially challenging in the elderly because of their diminished host capacity.
Imaging studies
The use of imaging studies is evolving but they typically involve initial radiographs followed by another modality. Radiographs may take up to 10 days from the onset of acute osteomyelitis to manifest any visible signs of infection, which are typically a permeative lesion or periosteal reaction (Figure 5.3).13 The earliest changes are usually seen in the adjacent soft tissues with swelling and potential loss of normal fat and muscle planes. It is not uncommon for an effusion to be present in adjacent joints as well. In order for bony changes on plain radiographs to be identified, the osteomyelitis must extend at least 1 cm and compromise 30–50% of the bone. Other specific changes include a periosteal reaction, bone lysis, loss of the osseous trabecular architecture, regional osteopenia, endosteal scalloping and eventual formation of a sequestrum and involucrum.17 The permeative changes in the bone have also been described as a ‘motheaten’ appearance. All of these radiographic characteristics are more difficult to diagnose in the elderly due to their relative osteopenia. This is particularly true in the trauma setting where hardware and callus formation can make these osseous changes more difficult to assess.
Computerized tomography (CT) remains superior to plain films and magnetic resonance imaging (MRI) is probably the best investigative modality for demonstrating signs of infection. Both tests can help identify the bony margins of a sequestrum and surrounding involucrum. CT scans are useful during staged debridements of osteomyelitis, especially for identifying an infected non-union after trauma. Software manipulation programs for both CT and MRI are making them more useful in the vicinity of metallic hardware and their use continues to evolve (Figures 5.4 and 5.5).
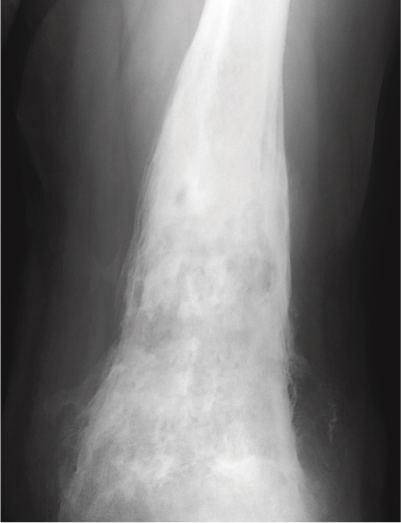
Figure 5.3 Plain radiograph displaying signs of osteomyelitis with characteristic changes of periosteal reaction and permeative ‘motheaten’ bone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








