2 Synovium
The synovium provides nutrients to cartilage and produces lubricants for the joint.
The intimal lining of the synovium includes macrophage-like and fibroblast-like synoviocytes.
Structure
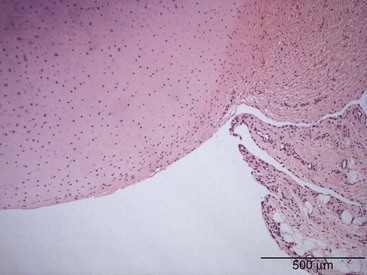
The synovium is a membranous structure that extends from the margins of articular cartilage and lines the capsule of diarthrodial joints, including the temporomandibular joint1 and the facet joints of vertebral bodies (Figure 2-1).2 The healthy synovium covers intra-articular tendons and ligaments, as well as fat pads, but not articular cartilage or meniscal tissue. Synovium also ensheathes tendons where they pass beneath ligamentous bands and bursas that cover areas of stress such as the patella and the olecranon. The synovial membrane is divided into general regions—the intima, or synovial lining, and the subintima, otherwise referred to as the sublining. The intima represents the interface between the cavity containing synovial fluid and the subintimal layer. No well-formed basement membrane separates the intima from the subintima. It is not a true lining, in contrast to the pleura or pericardium, because it lacks tight junctions, epithelial cells, and a well-formed basement membrane. The subintima is composed of fibrovascular connective tissue and merges with the densely collagenous fibrous joint capsule.
Synovial Lining Cells
The synovial intimal layer is composed of synovial lining cells (SLCs), which are arrayed on the luminal aspect of the joint cavity. SLCs, termed synoviocytes, are one to three cells deep, depending on the anatomic location, and extend 20 to 40 µm beneath the lining layer surface. The major and minor axes of SLCs measure 8 to 12 µm and 6 to 8 µm, respectively. The SLCs are not homogeneous and are conventionally divided into two major populations, namely, type A (macrophage-like) synoviocytes and type B (fibroblast-like) synoviocytes.3
Ultrastructure of Synovial Lining Cells
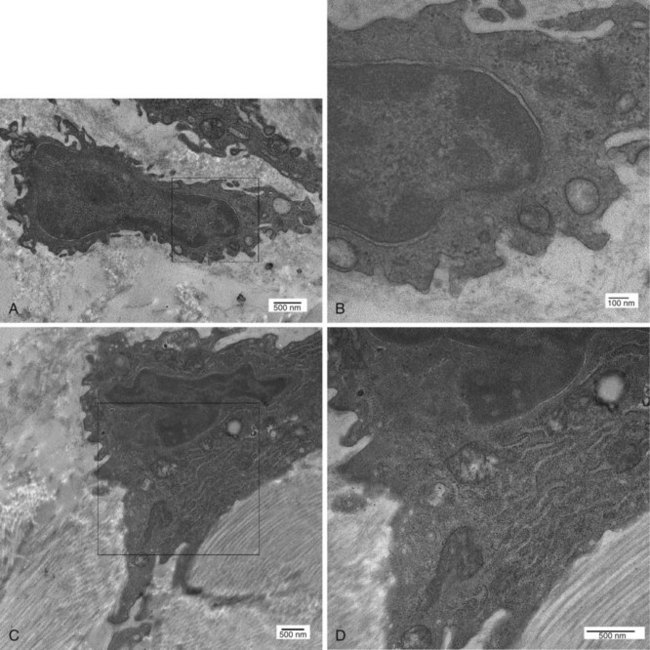
Transmission electron microscopic analysis shows that the intimal cells form a discontinuous layer, so that the subintimal matrix can directly contact the synovial fluid (Figure 2-2). The existence of two distinct cell types—type A and type B SLCs—was originally described by Barland and associates,4 and several lines of evidence, including animal models, detailed ultrastructural studies, and immunohistochemical analyses, indicate that these cells represent macrophages (type A SLCs) and fibroblasts (type B SLCs). Studies of SLC populations in a variety of species, including humans, have found that macrophages make up anywhere from 20% and fibroblast-like cells approximately 80% of the lining cell.5,6 The existence of the two cell types has been substantiated by similar findings in a wide variety of species, including hamsters, cats, dogs, guinea pigs, rabbits, mice, rats, and horses.6–14
Distinguishing different cell populations that form the synovial lining requires immunohistochemistry or transmission light microscopy. At an ultrastructural level, type A cells are characterized by a conspicuous Golgi apparatus, large vacuoles, and small vesicles, and they contain little rough endoplasmic reticulum, giving them a macrophage-like phenotype (Figure 2-3A and B). The plasma membrane of type A cells possesses numerous fine extensions, termed filopodia, which are characteristic of macrophages. Type A cells occasionally cluster at the tips of the synovial villi; this uneven distribution explains at least in part early reports that suggested type A cells were the predominant intimal cell type.4,8 However, the distribution is highly variable and can differ depending on the joint evaluated or even within an individual joint.
Type B SLCs have prominent cytoplasmic extensions that extend onto the surface of the synovial lining (Figure 2-3C and D).15 Frequent invaginations are seen along the plasma membrane; a large indented nucleus relative to the area of the surrounding cytoplasm is also a feature. Type B cells have abundant rough endoplasmic reticulum widely distributed in the cytoplasm, and the Golgi apparatus, vacuoles, and vesicles are generally inconspicuous, although some cells have small numbers of prominent vacuoles at their apical aspect. Type B SLCs are known to contain longitudinal bundles of different-sized filaments, supporting their classification as fibroblasts. Desmosomes and gap-like junctions have been described in rat, mouse, and rabbit synovium, but the existence of these structures in human SLCs has never been documented. Although occasional reports describe an intermediate synoviocyte phenotype, it is likely that these cells are functionally conventional type A or B cells.16,17
Immunohistochemical Profile of Synovial Cells
Synovial Macrophages
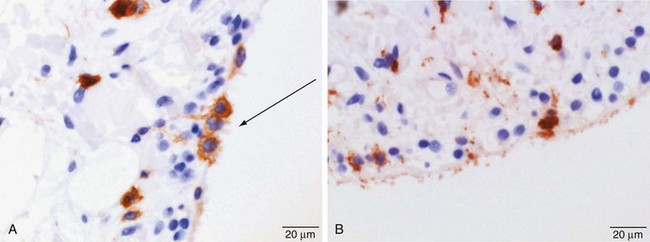
Synovial macrophages and fibroblasts express lineage-specific molecules, which can be detected by immunohistochemistry. Synovial macrophages express common hematopoietic antigen CD45 (Figure 2-4A); monocyte/macrophage receptors CD163 and CD97; and lysosomal enzymes CD68 (Figure 2-4B), neuron-specific esterase, and cathepsins B, L, and D. Cells expressing CD14, a molecule that acts as a co-receptor for the detection of bacterial lipopolysaccharide and expressed by circulating monocytes and monocytes newly recruited to tissue, are rarely seen in the healthy intimal layer, but small numbers are found close to venules in the subintima.18–24
The Fcγ receptor, FcγRIII (CD16), expressed by Kupffer cells of the liver and type II alveolar macrophages of the lung, is expressed on a subpopulation of synovial macrophages.25–27 The synovial macrophage population also expresses the major histocompatibility complex (MHC) class II molecule, which plays an important role in the immune response. More recently, the macrophages, which are responsible for the removal of debris, blood, and particulate material from the joint cavity and possess antigen processing properties, have been found to express the complement-related protein, Z39Ig, a cell surface receptor and immunoglobulin superfamily member involved in the induction of human leukocyte antigen (HLA)-DR and in implicated phagocytosis and antigen-mediated immune responses.28–30
Expression of the β2 integrin chains—CD18, CD11a, CD11b, and CD11c—varies; CD11a and CD11c may be absent or weakly expressed on a few lining cells.31,32 Osteoclasts, which are tartrate resistant and acid phosphatase positive and express the αvβ3 vitronectin and calcitonin receptors, do not appear in the normal synovium.
Synovial Intimal Fibroblasts
Synovial intimal and subintimal fibroblasts are indistinguishable by light microscopy. They generally are considered to be closely related in terms of cell lineage, but because of their different microenvironments, they do not always share the same phenotype. They possess prominent synthetic capacity and produce the essential joint lubricants hyaluronic acid (HA) and lubricin.33 Intimal fibroblasts express uridine diphosphoglucose dehydrogenase (UDPGD), an enzyme involved in HA synthesis that is a relatively specific marker for this cell type. UDPGD converts UDP-glucose to UDP-glucuronate, one of the two substrates required by HA synthase for assembly of the HA polymer.34 CD44, the nonintegrin receptor for HA, is expressed by all SLCs.32,35,36
Specialized intimal fibroblasts express many other molecules that might be expressed by the intimal macrophage population or by most subintimal fibroblasts, including decay-accelerating factor (CD55); vascular cell adhesion molecule-133,37–40; and cadherin-11.41,42 PGP.95, a neuronal marker, might be specific for type B synoviocytes in some species.43 Decay-accelerating factor, also expressed on many other cells (most notably erythrocytes) as well as bone marrow cells, interacts with CD97, a glycoprotein that is present on the surface of activated leukocytes, including intimal macrophages, and is thought to be involved in signaling processes early after leukocyte activation.44,45 In contrast, FcγRIII is expressed by macrophages only when they are in close contact with decay-accelerating factor–positive fibroblasts or decay-accelerating factor–coated fibrillin-1 microfibrils in the extracellular matrix.26
Cadherins are a class of tissue-restricted transmembrane proteins that play important roles in homophilic intercellular adhesion and are involved in maintaining the integrity of tissue architecture. Cadherin-11, which was cloned from rheumatoid arthritis synovial tissue, is expressed in normal synovial intimal fibroblasts, but not in intimal macrophages. The finding that fibroblasts transfected with cadherin-11 are induced to form a lining-like structure in vitro implicates this molecule in the architectural organization of the synovial lining.41,42,46 This suggestion is supported by the observation that cadherin-deficient mice have a hypoplastic synovial lining and are resistant to inflammatory arthritis.47 When fibroblasts expressing cadherin-11 are embedded in laminin microparticles, they migrate to the surface and form an intimal lining–like structure.48 If macrophage lineage cells are included in the culture, they can co-localize with fibroblasts on the surface. These data suggest that the organization of the synovial lining, including the distribution of type A and B cells, is orchestrated by the fibroblast-like synoviocytes.
β1 and β3 integrins are present on all SLCs, forming receptors for laminin (CD49f and CD49b), collagen types I and IV (CD49b), vitronectin (CD51), CD54 (a member of the immunoglobulin superfamily), and fibronectin (CD49d and CD49e). CD31 (platelet–endothelial cell adhesion molecule), a member of the immunoglobulin superfamily expressed on endothelial cells, platelets, and monocytes, is weakly expressed on SLCs.32
Turnover of Synovial Lining Cells
Proliferation of SLCs in humans is low, as is seen when normal human synovial explants, exposed to a pulse of 3H thymidine, cause SLCs to have a labeling index of approximately 0.05% to 0.3%49; this bears a striking contrast to labeling indices of approximately 50% for bowel crypt epithelium. Similar evidence of low proliferation has been found in the synovium of rats and rabbits. The proportion of SLCs expressing the proliferation marker Ki67 is between 1 in 2800 and 1 in 30,000, confirming the relatively slow rate of in situ proliferation.50 Proliferating cells are generally synovial fibroblasts22,51; this finding is consistent with the concept that type A synovial cells are terminally differentiated macrophages. Mitotic activity of SLCs is low in inflammatory conditions, such as rheumatoid arthritis—a condition associated with SLC hyperplasia. Some groups52 have reported only rare mitotic figures in rheumatoid arthritis synovium samples.
Apart from the knowledge that synovial fibroblasts proliferate slowly, little is known about their natural life span, recruitment, or mode of death. Apoptosis likely is involved in maintaining synovial homeostasis, but cultured fibroblast-like synoviocytes tend to be resistant to apoptosis, and very few intimal lining cells display evidence of completed apoptosis by ultrastructural analysis or by labeling for fragmented DNA. The paucity of normal synovium samples for evaluation and the rapid clearance of apoptotic cells could confound the analysis.53
Origin of Synovial Lining Cells
There is little doubt that the type A SLC population is bone marrow derived and represents cells of the mononuclear phagocyte system.4 Studies in the Beige (bg) mouse, which harbors a homozygous mutation that confers the presence of giant lysosomes in macrophages, have confirmed the bone marrow origin of these cells.54,55 Normal mice, bone marrow depleted through irradiation, were rescued with bone marrow cells obtained from the bg mouse. Electron microscopic analysis of the synovium from recipient animals revealed that type A SLCs contained the giant lysosomes of the donor bg mouse, and that these structures were never identified in type B cells. These findings provide powerful evidence that type A SLCs represent macrophages, that they are recruited from the bone marrow, and that they are a distinct lineage from type B SLCs.
1. The op/op mouse, a spontaneously occurring mutant that fails to produce macrophage colony-stimulating factor because of a missense mutation in the csf-1 gene,56–58 has low numbers of circulating and resident macrophage colony-stimulating factor–dependent macrophages, including those in the synovium.
2. Type A cells in rat synovium do not populate the joint until after the development of synovial blood vessels.22
3. Others have reported that type A SLCs were conspicuous around vessels in the synovium in neonatal mice.6
4. When synovial explants are placed in culture, the reduction in type A SLCs is explained in part by their migration into the culture medium—an observation that reflects the process of migration of macrophages into the synovial fluid in vivo.1,59
5. Macrophages are found around venules in disease states and constitute 80% of the intimal cells in inflammatory conditions such as rheumatoid arthritis.
Subintimal Layer
SLCs are not separated from the underlying subintima by a well-formed basement membrane composed of the typical trilaminar structure seen beneath epithelial mucosa. Nevertheless, most components of basement membrane are present in the extracellular matrix surrounding SLCs. These components include tenascin X, perlecan (a heparan sulfate proteoglycan), collagen type IV, laminin, and fibrillin-1.60,61 Of note is the absence of laminin-5 and integrin α3β3γ2, which are components of epithelial hemidesmosomes.62
The subintima is composed of loose connective tissue of variable thickness and variable proportions of fibrous/collagenous and adipose tissue, depending on the anatomic site. Under normal healthy conditions, inflammatory cells are virtually absent from the subintima, apart from a sprinkling of macrophages and scattered mast cells.63 Human synovial tissue is a rich source of mesenchymal stem cells, and although it is unknown which compartment contains this cell population, some cells have the ability to self-renew and differentiate into bone, cartilage, and fat in vitro—a phenomenon that reflects the ability of the cell to regenerate in vivo.64–66
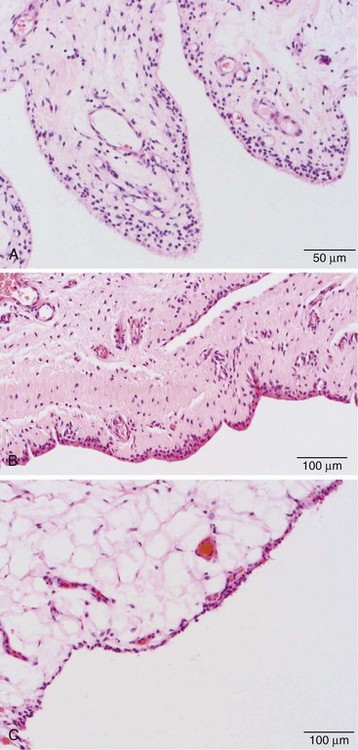
Three categories of subintima are well defined: areolar, fibrous, and fatty/adipose types. Under the light microscope, areolar-type subintima, the most commonly studied, generally is found in larger joints in which there is free movement (Figure 2-5A). It is composed of fronds with a cellular intimal lining and loose connective tissue in the subintima, with little in the way of dense collagen fibers, and a rich vasculature. The fibrous subintima is composed of scant dense fibrous, poorly vascularized connective tissue and has an attenuated layer of SLCs (Figure 2-5B). The adipose type contains abundant mature fat cells and has a single layer of SLCs. This is seen more commonly with aging and in intra-articular fat pads (Figure 2-5C).
The subintima contains collagen types I, III, V, and VI; glycosaminoglycans; proteoglycans; and extracellular matrices including tenascin and laminins. Integrin receptors for collagens, laminin, and vitronectin are absent or at best weakly expressed by subintimal cells. In contrast, receptors for fibronectin (CD49d and CD49e) are detected, and CD44, the HA receptor, is strongly expressed in most subintimal cells. β2 integrins are largely limited to perivascular areas, particularly in the subintimal zone, as is CD54.67
Subintimal Vasculature
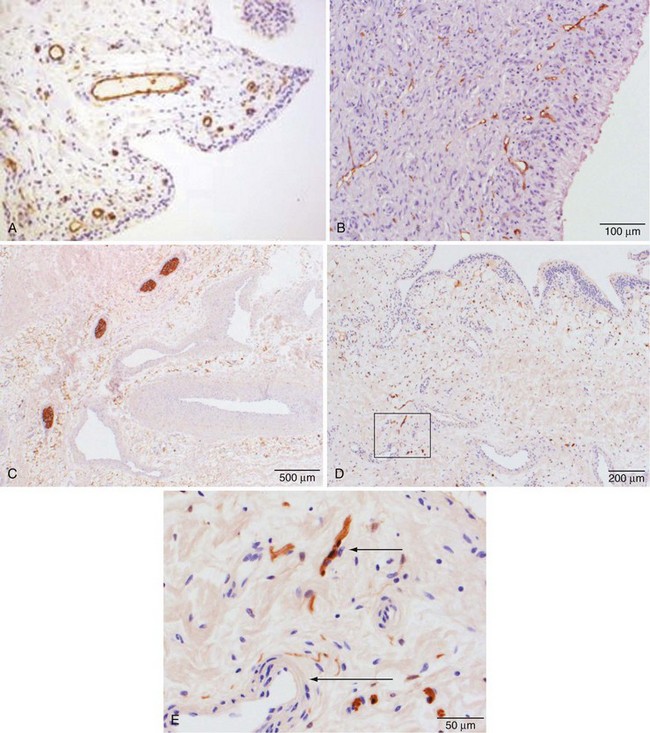
Vascular endothelial lining cells express CD34 and CD31 (Figure 2-6A). They also express receptors for the major components of basement membrane, including laminin and collagen IV, and the integrin receptors CD49a (laminin and collagen receptors), CD49d (fibronectin receptor), CD41, CD51 (vitronectin receptor), and CD61 (the β3 integrin subunit). Endothelial cells express CD44, the HA receptor, and CD62, P-selectin, which acts as a receptor that supports binding of leukocytes to activated platelets and endothelium. They are only weakly positive in uninflamed synovium, however, for expression of CD54, intercellular adhesion molecule-1, a receptor for β2 integrins expressed by many leukocytes. The endothelial cells of capillaries in the superficial zone of the subintima are strongly positive for HLA-DR expression by immunohistochemistry, whereas cells in the larger vessels in the deep aspect of the membrane are negative.32,34
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree