Soft tissue injuries
Greater trochanter pain syndrome
Anterior cruciate ligament tears in older patients
Achilles tendon injury in older patients
INTRODUCTION
Soft tissue injuries are common not only in young and active patients but also in older individuals. Rotator cuff injury, greater trochanter pain syndrome, anterior cruciate ligament rupture and Achilles tendon injury are debilitating problems both for older people who practice sporting activities and in everyday activities. In this chapter, these frequent conditions are described and evidence based treatment strategies are discussed.
ROTATOR CUFF INJURY
Rotator cuff tears (RCTs) are a frequent source of shoulder pain and disability in older patients. Since the first description of an RCT by Smith1 in the London Medical Gazette in 1834, a wide variation in the prevalence of RCTs has been reported. Many studies have been conducted in symptomatic and asymptomatic patients, and imaging and cadaveric studies have been performed. Cadaver studies estimate the prevalence of full thickness tears ranging from 5% to 30%.2 In 2006, a review of the cadaveric and imaging prevalence of RCTs3 showed an overall prevalence of 23% in 4629 cadaveric shoulders. The prevalence of RCTs increases linearly with age from the third decade, increasing from 33% in the 40s to 55% in the 50s.4
Pathogenesis
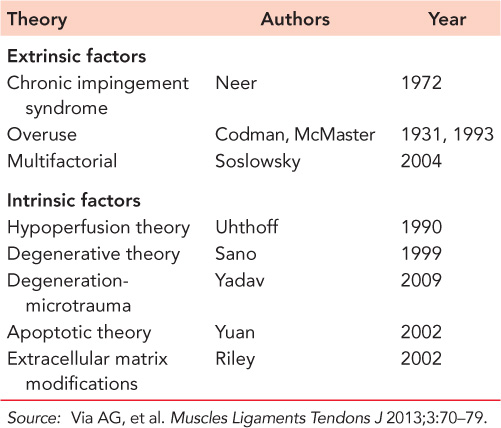
The pathogenesis of RCTs is multifactorial and is still not completely clear.4 Many theories have been proposed, and tears are traditionally classified into ‘extrinsic’ and ‘intrinsic’ (Table 44.1). Chronic impingement, as described by Neer in his chronic impingement syndrome theory, is the best known extrinsic pathological factor in RCTs.5 Excessive load, repetitive load or loads applied from different directions have been implicated in the process of tendinopathy. Other theories include localized hypoxia produced by tensile load, hyperthermic injury as the tendon heats up with exercise, tenocyte apoptosis, and cytokines or proteolytic enzymes released as a result of applied stress. The release of nitrous oxide has also been implicated in the tendinopathy process. Currently, RCTs are considered to be of multifactorial etiology, and the relative contributions of these factors remain to be determined.
Any process that impairs tissue healing may be implicated in rotator cuff disease. Nicotine exerts deleterious effects on tendon healing,6 and smokers are less likely to respond favorably to cuff repair operations, with reduced postoperative function and satisfaction compared to non-smokers.7 In the current literature, factors such as patient age, sex and fatty muscle infiltration are highly correlated to the presence of RCTs and the prevalence of recurrence of tears.4
Many studies emphasize the importance of extracellular matrix (ECM) for the homeostasis of connective tissue. ECM is the substrate to which cells adhere, migrate and differentiate. ECM imparts information to cells and tissues by providing cell-binding motifs in its own proteins or by presenting growth factors and morphogens to the cells. Physiological and pathological modifications of the ECM seem to be the most important intrinsic factors involved in tendinopathies and tendon ruptures.8 Transglutaminases (TGs) have been implicated in the formation of hard tissue development, matrix maturation and mineralization and have been shown to be down-regulated in human supraspinatus tendon ruptures.9
Table 44.1 Extrinsic and intrinsic theories about the pathogenesis of rotator cuff tears
The roles of hormonal and metabolic diseases have recently been investigated.10 The relationship between thyroid disorders and shoulder pain has been suspected since the late 1920s11 but has not been systematically investigated. The thyroid hormones, T3 and T4, play an essential role in the development and metabolism of many tissues and organs. Thyroxine is important for both collagen synthesis and ECM metabolism. A recent study demonstrated that thyroid hormone nuclear receptors are present in healthy and pathologic rotator cuff tendons, and that, in vitro, thyroid hormones enhance tenocyte growth and counteract apoptosis in healthy tenocytes isolated from tendon in a dose- and time-dependent manner.12 Hypothyroidism causes accumulation of glycosaminoglycans in the ECM, which may, in turn, predispose to tendon calcification.10 Diabetes is also a risk factor for RCTs.13 In a study on asymptomatic subjects, age related rotator cuff tendon changes were more common in diabetic subjects.14 Diabetic patients show a restricted shoulder range of motion, a higher prevalence of recurrent tears after surgical repair and a higher rate of complications and infections after open and arthroscopic repair of RCTs.15 An association between obesity and RCTs has also been proposed.16
Physical examination
The clinical diagnosis is not always easy. Painful conditions of the long head of the biceps or the acromioclavicular joint may result in a high false positive rate. The clinical presentation of rotator cuff pathology is extremely variable. A recent review concluded that RCTs are frequently asymptomatic.7 Such variation in clinical features remains unexplained. Physical examination should include inspection, palpation, the evaluation of active and passive range of motion and the execution of strength and provocative tests. Many specific clinical tests are available to test the muscles forming the rotator cuff. Patients with shoulder pain who test positive for supraspinatus weakness, weakness in external rotation and impingement have 98% chance of having an RCT. If none of these clinical features are present, the chance of having a tear decreases to 5%.4
Rotator cuff healing
Tendon healing is a complex and well-orchestrated series of physiological events involving synthesis, migration and degradation of ECM components. The ability of tendons to heal is controversial: tendon tissue can repair but not regenerate.13 Tissue adjacent to an RCT (2.5 mm) appears to be histologically viable in both microvasculature and cellular synthesis of type I procollagen. Therefore, many authors advise avoiding wide excision of the edges of the torn rotator cuff before reattachment.4 This is supported by the observation of Jost et al.17 who, in a long-term follow-up after structural failure of rotator cuff repairs, observed compelling evidence that small reruptures have a potential to heal.
It is very important to provide the tendon with the best conditions to heal. The subacromial bursa seems to play an important role. In normal conditions, the subacromial bursa has three functions. It facilitates gliding between two layers of tissue, provides blood supply to the cuff tendons and provides cells and vessels to aid healing after surgical repair. Mobilization after repair promotes cellular activity, improves tensile properties and enhances gliding function. A fine balance exists between the progression of tendon healing and postoperative mobilization.
Treatment of rotator cuff tears
Both arthroscopic and mini-open techniques are effective for rotator cuff repair, but there is still considerable debate over the benefits of these procedures. Many improvements have been made since the first completely arthroscopic rotator cuff repair described by Johnson,18 and arthroscopists state that the main advantages of all-arthroscopic repairs are less postoperative pain and shorter rehabilitation time. However, when the outcome of arthroscopic and mini-open repair are compared, no significant differences are found. Functional outcome, pain, range of motion and complications do not significantly differ between all arthroscopically repaired patients and those treated with mini-open repair in the first year after surgery.19 Pearsall et al.20 and Kim et al.,21 in retrospective studies, found no differences in outcome for small and medium sized tears of the rotator cuff at mid-term follow-up. A systematic review showed decreased pain at short-term follow-up in patients treated arthroscopically.22 However, massive tears remain difficult to treat arthroscopically, and the mini-open surgical technique could be a good choice in the elderly patient. Another difference between the two different methods is the re-tear rate, which is higher in patients treated arthroscopically at 24-month follow-up.23 In reality, there is no evidence about which is the best treatment.
Irreparable RCTs in elderly patients are a challenging problem. There are several treatment options, but determining the correct treatment for each patient is difficult. Reverse shoulder arthroplasty (RSA) is an emerging solution for these patients in whom anatomic shoulder arthroplasty or hemiarthroplasty has failed. Many mid-term studies showed early encouraging results, and the first long-term studies show good survivorship. Favard et al.24 retrospectively reviewed 527 arthroplasties in 506 patients and found 89% survivorship at 10 years. Similar results have been reported by Guery et al.,25 who demonstrated deteriorating function and increasing pain after 6 years. Boileau et al.26 reported improved shoulder function and restored active elevation in patients with cuff-deficient shoulders treated with RSA. Supported by these encouraging results, original indications for RSA were expanded. Originally, the reverse shoulder prostheses were designed for rotator cuff arthropathy, and massive RCTs with arthritis and massive irreparable RCTs were considered appropriate indications. Recently, displaced three- or four-part neck of humerus fractures in older patients, RCTs with fatty infiltration of infraspinatus or subscapularis muscles, sequelae of fracture, rheumatoid arthritis, revision arthroplasty and tumors are becoming common indications for RSA.27 RSA combined with latissimus dorsi and teres major tendon transfer has been developed to improve external rotation and spatial control in patients with no functional teres minor and infraspinatus with early promising results.28 However, a high rate of complications is still reported after RSA and progressive functional and radiographic deterioration continue to be of concern.29 Scapular notching is one of the most important complications of RSA.25
Future perspectives
Several strategies have been proposed to enhance tendon healing. Recently research focused on regenerative therapies such as growth factors (GFs) and plasma rich platelet (PRP) which have become a popular treatment for tendon injuries. However, the efficacy of PRP treatment is highly controversial. In vitro studies showed that the addition of PRP to human tenocytes resulted in cell proliferation, collagen deposition, well-ordered angiogenesis and improved gene expression for matrix degrading enzymes and endogenous growth factors.30,31 More recently, two studies demonstrated that PRP induced in vitro tendon mesenchymal stem cells (T-MSCs) to differentiate into active tenocytes, and that PRP has an anti-inflammatory function by suppressing the levels of prostaglandin E (PGE) biosynthetic pathway components (COX-1, COX-2 and PGE synthase-1 [PGES-1] expression) and PGE2 production.32 These results have important clinical implications because high levels of PGE2 cause pain, decrease cell proliferation and collagen production and induce degenerative changes in rabbit tendons.33 But the same authors also reported that, even if PRP is able to induce the differentiation of T-MSCs into tenocytes under regular culture conditions, PRP injection in clinics may not be able to effectively reverse the degenerative conditions of late-stage tendinopathy.34
However, clinical studies do not report any substantial benefit using PRP for rotator cuff lesions. A few studies demonstrated that the application of PRP for large to massive rotator cuff repairs significantly improved structural outcomes with a decreased re-tear rate.35 Gumina found that the platelet-leukocyte membrane improved structural repair integrity, but without improvement in functional outcome.36 Other works did not report better results with the use of PRP. Randelli et al.37 found that PRP reduces pain in the short term, making it possible to mobilize the patient earlier, but they did not find long-term improvement in functional scores. No differences in rotator cuff healing or improvements in function were found at 1-year follow-up.38 No statistically significant difference in the recurrent tear rate between patients treated with or without the addition of PRP was found.38 Castricini et al.39 performed a randomized double blind controlled trial with 88 patients and there were no statistically significant differences at 16-month follow-up. They stated that the study does not support the use of PRP in small to medium sized RCTs. There results were confirmed in a further RCT by Rodeo et al.40
GREATER TROCHANTER PAIN SYNDROME
Lateral hip pain is a debilitating condition characterized by pain at or around the greater trochanter, which is the site of confluence of three bursae, the hip abductor-lateral thigh muscles and the iliotibial tract. It was originally described as trochanteric bursitis, but advanced imaging and surgical findings did not evidence a real bursal involvement and instead showed different disorders such as insertional tendinopathy, tendon tears or avulsion of the gluteus medius and gluteus minimus tendons.41,42 External coxa saltans (snapping hip) is also related to greater trochanter pain. For these reasons, the term greater trochanteric pain syndrome (GTPS) is now used to better define this clinical condition.41
The incidence of GTPS is reported to be approximately 1.8 per 1000 patients per year, and it is more frequent in women (female:male ratio of 4:1) between 40 and 60 years old. It has been reported to affect 10–25% of the general population, and up to 35% in patients with leg length discrepancy and low back pain.43 It is particularly frequent in road runners, exposed to increased friction over the iliotibial tract over the greater trochanter, but it occurs also in sedentary older patients. Acute trauma, osteoarthritis, rheumatoid arthritis, lumbosacral disorders and infections (especially tuberculosis) have to be excluded.
The diagnosis of GTPS is usually based on clinical findings. Plain radiographs are performed in all patients to exclude concomitant hip or knee joint disease. Calcification may be detected at the site of insertion to the greater trochanter. MRI is useful to recognize partial and full tears of the gluteus medius and minimus tendons, tendon calcification and fatty muscle degeneration.
The optimal management for GTPS remains unclear. Conservative measures, including relative rest, nonsteroidal anti-inflammatories, ice, and supervised stretching and strengthening exercises are usually effective as the first-line management of GTPS. Home training programs seem to be effective, and good results and improvement in symptoms have been reported in 41% of patients at 4-month follow-up and in 80% of patients at 15 months.42 The correction of training errors and modifying physical activities is also important, while leg length discrepancy was recently confirmed to be a risk factor for GTPS. Local corticosteroid injections are widely used in clinical practice, but there is no conclusive evidence on their effectiveness. Small observational studies suggest that corticosteroid injections provide good short-term outcomes, but symptom recurrence and incomplete pain relief are not uncommon. Furthermore, fluoroscopically assisted injections of corticosteroid and local anaesthetics have not resulted in better treatment outcomes.42 Laser therapy and shock wave therapy are useful for the treatment of GTPS. A randomized controlled trial comparing different non-operative treatments reported that, even though the results of corticosteroid injections were significantly better than those of home training or shock wave therapy at 1-month follow-up, their effect quickly declined after 1 month. At 15-month follow-up, home training and shock wave therapy were more effective than corticosteroid injection, with success rates of 74%, 80% and 48%, respectively.44 The authors concluded that the role of corticosteroid injection for GTPS should be reconsidered because it is significantly less successful than home training and shock wave therapy at long-term follow-up.
If conservative treatment fails, surgery is indicated. Several operative procedures, open and endoscopic, have been described for patients not responding to conservative treatment. In a study by Slawski and Howard, five active patients (seven hips) received longitudinal release of the iliotibial band and excision of the subgluteal bursa.45 All patients were satisfied with the surgical results and returned to their pre-injury sporting level. Pain reduction has been reported after open trochanteric reduction osteotomy, tendon repair and reattachment of the medius gluteus. Endoscopic bursectomy has also been proposed with early improvements at 1-month follow-up. Minimally invasive procedures such as endoscopic repair techniques provided good short-term outcomes,2 but further larger studies, with longer follow-up, are needed.46
ANTERIOR CRUCIATE LIGAMENT TEARS IN OLDER PATIENTS
The incidence of anterior cruciate ligament (ACL) tears in the United States is about 200,000 per year, with at least 50% of these patients undergoing arthroscopic reconstruction. Although this procedure may reduce the progression of knee osteoarthritis in chronic ACL deficiency, the management of knee instability in older patients is debated. Satisfactory functional outcomes have been reported in patients older than 50 undergoing conservative management at long-term follow-up.47 However, reduction in recreational activity level and increased chronic instability impair physical activities in older patients with a high functional activity level, and ACL reconstruction may be indicated.48 The management of older patients with ACL tears depends on several specific factors, including age, occupation and desired activity level. In less active people with sedentary lifestyles, conservative management consisting of physical therapy and activity modification can provide successful outcomes, but surgery may be indicated in active patients participating in jumping or pivoting sports.47
Although the management of older patients with ACL insufficiency remains controversial, in the last few years surgical management of ACL deficiency has been increasingly advocated. A study comparing pre- and postoperative status showed that patients over 50 who underwent ACL reconstruction experienced improved postoperative clinical outcomes. Even if older patients show a lower return rate to pre-injury sport activity level, they seem to be subjectively more satisfied with the overall results than younger patients. Surgical expectations should be tailored to age and activity level. Surgical management in healthy subjects older than 40 years to prevent further secondary injuries and return to pre-injury sport activity performance status has been advocated by some authors.49
Even if age and time from injury to surgery have been considered as risk factors for osteoarthritis, they are not absolute contraindications to surgical management, and age itself is not a contraindication to ACL surgery. When faced with patients with ACL instability, physiological age, condition of the knee at the time of examination, life expectancy and activity levels are probably more important than chronologic age. The optimal treatment in adult patients with ACL tears should be planned after careful consideration of the patient’s characteristics, their desire to return to activity and knee-specific comorbidities, especially meniscal pathology or osteoarthritis.
ACHILLES TENDON INJURY IN OLDER PATIENTS
Tendinopathy of the main body of the Achilles tendon (AT) affects both athletic and sedentary patients, and about 30% of patients do not participate in sports activities.50 Acute AT rupture is a serious injury, with an incidence ranging from 6 to 18 per 100,000 subjects per year. Most (75%) acute ruptures occur during recreational activities in men between the ages of 30 and 40, and 25% of ruptures occur in sedentary patients.51
The pathogenesis of AT injuries is not completely clear. Metabolic diseases, such as diabetes mellitus,52 hypercholesterolemia and obesity, seem to play a role.53 Hyperglycaemia may be a risk factor for tendinopathy because of non-enzymatic glycosylation processes which change collagen cross-links.54
Management of acute ruptures of the AT is still controversial. Operative management provides earlier functional rehabilitation, less calf atrophy and stronger pushoff than non-surgical treatment. Surgical treatment ensures a lower rerupture rate compared to non-operative treatment, which has been reported in up to 13% of patients treated conservatively.55 A recent review article showed that surgical treatment results in higher costs and a 20-fold higher rate of complications, such as wound problems and superficial or deep infections, than conservative treatment.56 On the other hand, recent well-conducted randomized controlled trials showed that conservative and open surgery management produce, in an unselected population, similar functional results. Willits et al. showed acceptable and similar outcomes in patients treated with accelerated functional rehabilitation for acute AT ruptures compared to patients who received operative repair.57 However, the first group experienced a higher rerupture rate. Non-operative management using functional bracing with early mobilization has similar outcomes compared to open surgical treatment with regard to rerupture rate, range of motion and calf circumference.58 The major advantage of conservative treatment is a lower rate of complications.59 The risk of complications following surgery was 3.9-fold than in non-surgically treated patients, with an absolute risk increase of 15.8%.
Other concerns when dealing with elderly patients with AT rupture include the high incidence of comorbidities in this age group. Comorbidities can make it difficult for the patients to return to their previous state of activity and immobility in the elderly may progressively impair their general health status.
Minimally invasive AT repair has been successfully used to avoid these complications and it is becoming a well-accepted treatment. It provides many advantages, such as less iatrogenic damage to normal tissues, less postoperative pain, accurate opposition of the tendon ends, minimized surgical incisions and improved cosmesis. A recent systematic review reported a rate of superficial infections of 0.5% and 4.3% after minimally invasive and open surgeries, respectively. Deep infections did not occur in subjects who received minimally invasive repair.56 Shorter hospitalization and average time to return to work were also found in the minimally invasive group. The indications were grossly comparable, and the functional outcomes were not significantly different between minimally invasive and open surgery. Although sural nerve injury has been reported as a potential complication of this kind of surgery, new techniques have minimized the risk of sural nerve damage. Good results have been reported in 27 patients with a mean age of 73 years.60 All patients were able to bear weight fully on the affected limb by the eighth postoperative week. The rerupture rate was 7%, superficial infection managed with oral antibiotics occurred in 11% of cases, and 11% of patients experienced hypaesthesia over the area of distribution of the sural nerve which resolved over 6 months in most cases. The authors concluded that percutaneous repair of the AT is a suitable option for patients older than 65 because it reduces the risk of rerupture compared to non-operative treatment, produces lower risk of other complications and provides similar outcomes compared to percutaneous repair in younger patients. Encouraging results have also been reported in diabetic patients, with a rate of superficial infection of 20%.61 Hence, mini-invasive techniques are a proper alternative for AT repair in older patients, in particular in patients with comorbidities such as diabetes or vascular diseases with higher risk of infection and wound complications.
Percutaneous Achilles tendon repair: surgical technique
A 1-cm transverse incision is made over the defect using a size 11 blade. Four longitudinal stab incisions are made lateral and medial to the tendon 6 cm proximal to the palpable defect. Two further longitudinal incisions on either side of the tendon are made 4–6 cm distal to the palpable defect. Forceps are then used to mobilize the tendon from beneath the subcutaneous tissues. A 9 cm Mayo needle is threaded with two double loops of Number 1 Maxon, and this is passed transversely between the proximal stab incisions through the bulk of the tendon (Figure 44.1). The bulk of the tendon is surprisingly superficial. The loose ends are held with a clip. In turn, each of the ends is then passed distally from just proximal to the transverse Maxon passage through the bulk of the tendon to pass out of the diagonally opposing stab incision. A subsequent diagonal pass is then made to the transverse incision over the ruptured tendon. To prevent entanglement, both ends of the Maxon are held in separate clips. This suture is then tested for security by pulling with both ends of the Maxon distally. Another double loop of Maxon is then passed between the distal stab incisions through the tendon (Figure 44.2), and in turn through the tendon and out of the transverse incision starting distal to the transverse passage (Figure 44.3). The ankle is held in full plantar flexion, and in turn opposing ends of the Maxon thread are tied together with a double throw knot, and then three further throws before being buried using the forceps. A clip is used to hold the first throw of the lateral side to maintain the tension of the suture. We use 3-0 Vicryl sutures to close the transverse incision and Steri-Strips to close the stab incisions (Figure 44.4). A non-adherent dressing is applied. A full plaster cast is applied in the operating room with the ankle in physiologic equinus. The cast is split on both medial and lateral sides to allow for swelling. The patient is discharged on the same day of the operation.
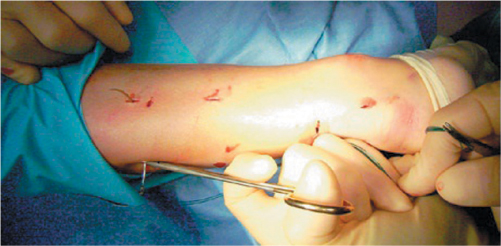
Figure 44.1 A 9 cm Mayo needle is threaded with two double loops of Number 1 Maxon, and this is passed transversely between the proximal stab incision through the bulk of the tendon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








