77 Psoriatic Arthritis
Psoriatic arthritis is a member of the spondyloarthropathy family and may be defined as an inflammatory arthropathy associated with psoriasis and usually negative for rheumatoid factor. Until the 1950s, an inflammatory arthritis occurring in the presence of psoriasis was thought to represent rheumatoid arthritis (RA) occurring coincidentally with psoriasis. Based primarily on clinical and radiologic grounds and using the rheumatoid factor, the distinction between RA and psoriatic arthritis became gradually accepted. Wright described the classic clinical features in 1959, and together with his colleague Moll, he published his classification criteria in 1973.1,2 These criteria have remained until recently the simplest and the most frequently used in clinical studies. The American Association of Rheumatism included psoriatic arthritis as a distinct clinical entity in the classification of rheumatic diseases for the first time in 1964.3
Epidemiology
Epidemiologic studies have supported the concept that psoriatic arthritis is a unique disease entity separate from RA. The prevalence of inflammatory arthritis is increased among patients with psoriasis, ranging from 7% to 25% compared with a general population estimate of 2% to 3%. The prevalence of psoriasis among subjects with arthritis also is increased at 2.6% to 7% compared with a general population estimate of 0.1% to 2.8%.4
Psoriasis affects about 2% of the population. The prevalence varies, with 5% to 10% of Russians and Norwegians affected, and only 0% to 0.3% of West Africans or Native Americans affected.5 Onset of psoriasis may occur at any age, but it most frequently peaks in the 20s. Although no gender predilection has been reported, a genetic predisposition has been noted.
Among patients with psoriasis, 7% to 42% develop arthritis. This figure varies so widely in part because of a lack of widely accepted diagnostic criteria; it also varies according to which population is being studied. The exact prevalence and incidence of psoriatic arthritis are unknown. The reported prevalence of psoriatic arthritis ranged from 0.056% to 0.28% in a large population-based study in the United States.6 Cases were defined as patients who reported a “physician diagnosis” of psoriasis and psoriatic arthritis. Prevalence was calculated at 0.25% (95% confidence interval, 0.18% to 0.31%). Kay and colleagues7 did a prevalence study in northeast England to evaluate records from six general practices; 81 of 772 psoriasis subjects had an inflammatory arthritis with a prevalence of 0.28%. The reported incidence of psoriatic arthritis has varied from 3 to 23 cases per 100,000. Data from Rochester, New York, have shown an incidence rate of 6.59 per 100,000, whereas in Finland, 16 new cases of psoriatic arthritis were identified in a population of 87,000, yielding a mean incidence rate of 23 per 100,000.8,9 Using the Icelandic genealogy database, risk ratios (RRs) for developing psoriatic arthritis spanning five generations were estimated.10 Results confirmed a strong and complex genetic component with a significant risk ratio up to the fourth-degree relatives of psoriatic arthritis patients, as well as an important environmental contribution.
Clinical Features
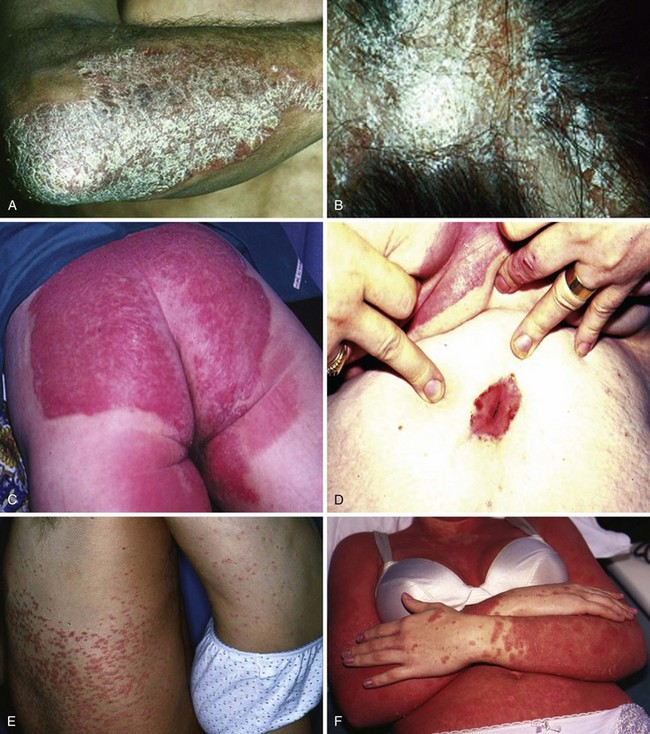
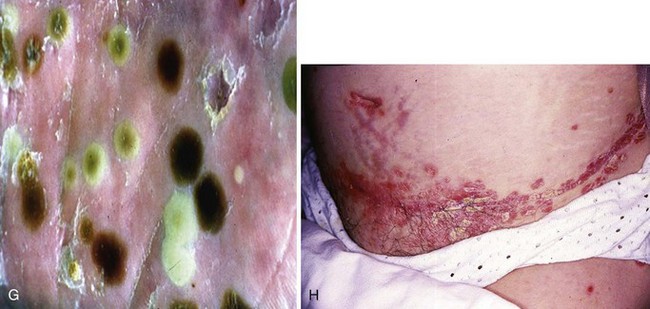
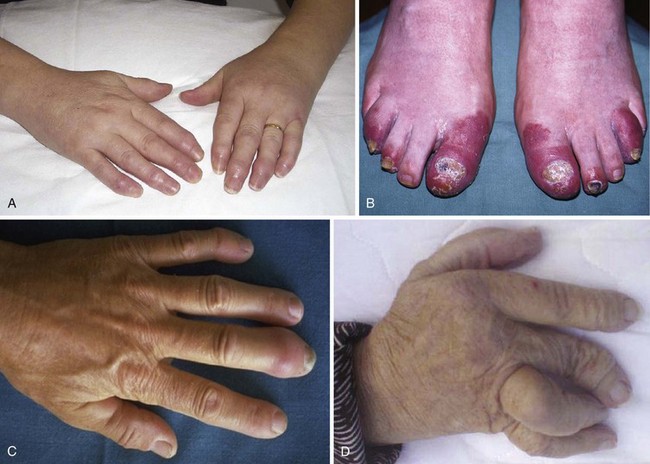
Plaque psoriasis or psoriasis vulgaris is the most common skin phenotype in patients with psoriatic arthritis. Other patterns of skin involvement may be seen (Figure 77-1). Although arthritis usually develops in a setting of an established diagnosis of psoriasis, some patients may be unaware that they have psoriasis, or psoriasis may develop after the onset of arthritis in approximately 15% of cases.11 In a clinical study, the incidence of development of arthritis in patients with psoriasis remained constant (74 per 1000 person-years), while the prevalence increased with time since diagnosis of psoriasis, reaching 20.5% after 30 years.12 If a patient presents with the classic articular manifestations of psoriatic arthritis but does not volunteer psoriasis or the presence of a rash, it is incumbent on the physician to examine the patient’s skin carefully, including the scalp and nails, because psoriasis frequently lurks in such areas. Examples of nail dystrophic changes are shown in Figure 77-2.
Although a more recent U.S. study suggests that the prevalence of psoriatic arthritis among psoriasis patients increases with psoriasis severity,6 in clinical practice, little relationship has been observed between severity of skin involvement and severity of arthritis. In one prospective study, only 35% of patients reported that their skin and joint components flared at the same time.11 Other systemic features of joint inflammation are common in patients with psoriatic arthritis, including stiffness after rest and fatigue. In one study of fatigue, a number of factors, including disease activity, physical disability, pain, and psychologic distress, contributed to fatigue, with comorbid fibromyalgia and hypertension further adding to the challenge.13 Compared with other core outcome measures, fatigue has been found to be an independent outcome measure that is sensitive to change.14
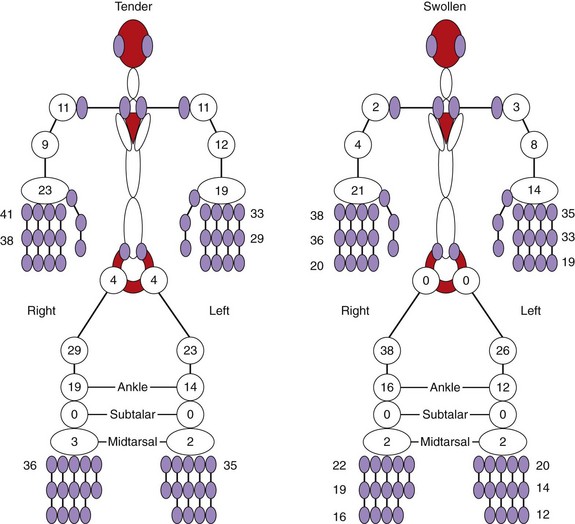
Patients with psoriatic arthritis present with symptoms and signs of joint, entheseal, or spinal inflammation. The joints involved at presentation in 129 early psoriatic arthritis patients are shown in Figure 77-3. In one of their seminal papers on psoriatic arthritis, Wright and Moll15 described five clinical patterns of psoriatic arthritis (Figure 77-4):
These classification criteria are the most commonly quoted, although many alternative criteria have been proposed. Variability in the definition of terms has led to differences in the reported frequency of psoriatic arthritis in subsets among the different studies. The pattern of joint involvement is not fixed; the patient’s disease may fluctuate and may be influenced by treatment. In a study of 129 patients with early psoriatic arthritis, 53 of 77 initially classified as polyarticular were reassessed at 2 years; 26 of 53 (49%) patients were subsequently classified as oligoarticular, 19 of 53 (36%) remained classified as polyarticular, and 12 of 53 (23%) were in remission.16
The Classification of Psoriatic Arthritis (CASPAR) study has included in the analysis a breakdown of disease pattern subtypes.17 This multicenter study included data on 588 psoriatic arthritis cases and 536 controls. In contrast to the original Moll and Wright paper, but similar to many subsequent publications, approximately 63% of patients had polyarticular joint involvement compared with 13% with oligoarticular disease. The other patterns of joint involvement described by Moll and Wright occurred much less commonly. Predominant DIP disease was found in less than 5%, but DIP involvement can occur in any of the subtypes. Predominant spondyloarthritis is uncommon, although spinal involvement may be found in 40% to 70% of psoriatic arthritis cases, depending on whether or not radiographs are taken.18 Risk factors for spinal involvement include severe peripheral arthritis and HLA-B27.19 Finally, arthritis mutilans, a destructive form of arthritis associated with flail joints, is rare, although more patients may develop this form of joint involvement with time if their disease is not properly controlled.
Features that are typical of psoriatic arthritis, including dactylitis and enthesitis, are helpful in diagnosis. Dactylitis, which is characterized by a sausage-shaped swelling of the fingers or toes (see Figure 77-4B), may be found in 29% to 33.5% of psoriatic arthritis patients at first presentation, and 48% may have an episode of dactylitis during follow-up.16,20 Ultrasound and magnetic resonance imaging (MRI) studies have shown that joint and tenosynovial inflammation are prominent in involved digits.21,22 Enthesitis, inflammation at tendon and ligament insertion into bone, is a feature of all of the spondyloarthropathies and may be a presenting feature in psoriatic arthritis. Overall, enthesitis is found in 38% of patients at presentation.16 The most common entheseal sites involved are the Achilles and plantar fascia insertions. Other sites include the insertions of the quadriceps and patellar tendons, the iliac crest, the rotator cuff, and the epicondyles at the elbow. Patients have reported pain at these sites, with tenderness and sometimes swelling noted on examination. Entheseal involvement may be asymptomatic, and ultrasound is more sensitive than clinical palpation. Often spurs are detected on x-ray, although spurs are not always associated with symptoms.
With the obvious exception of psoriasis and nail dystrophic change, extra-articular disease is less common in psoriatic arthritis than in RA. Iritis or uveitis occurs in 7% to 18%, more bilateral than in ankylosing spondylitis, but is usually found in patients with spinal involvement.11,23 Numerous studies have suggested that psoriatic arthritis patients have a higher prevalence of inflammatory bowel disease, sometimes asymptomatic and detected only on biopsy specimen.24,25 Whether this inflammatory bowel disease is coincidental or is possibly related to medication effects remains to be clarified. Distal limb edema or lymphedema may occur more commonly in psoriatic arthritis; one case-control study found it in 21% of psoriatic arthritis patients compared with 4.9% of controls (see Figure 77-4A).26 Finally, amyloid is rare but is described in psoriatic arthritis.
Differential Diagnosis
Certain articular features if present are useful in distinguishing psoriatic arthritis from RA, including dactylitis, DIP involvement, and inflammation at entheseal sites (Table 77-1; see Figure 77-4). In addition, inflammatory-type back pain or sacroiliitis on plain x-ray or MRI should raise the suspicion of psoriatic arthritis because spinal involvement is uncommon in RA. The absence of rheumatoid nodules or other systemic features common to RA can be another useful differentiating feature.
Table 77-1 Clinical Features That Distinguish Psoriatic Arthritis from Rheumatoid Arthritis
| Psoriatic Arthritis | Rheumatoid Arthritis | |
|---|---|---|
| Psoriasis | + | − |
| Symmetric | + | ++ |
| Asymmetric | ++ | + |
| Enthesopathy | + | − |
| Dactylitis | + | − |
| Nail dystrophy | + | − |
| Human immunodeficiency virus association | + | − |
Distinguishing psoriatic arthritis from other spondyloarthropathies is also important. Dactylitis may be a feature in reactive arthritis, where a palmoplantar pustular rash (keratoderma blennorrhagicum) may be clinically and histologically indistinguishable from pustular psoriasis (see Figure 77-1G). In relation to spinal involvement, sacroiliitis may be unilateral more frequently, and the spinal changes on plain radiography may be more asymmetric in psoriatic arthritis than with classic ankylosing spondylitis. Finally, crystal-associated arthropathies occasionally can confuse, especially with monoarticular disease, and are best distinguished by synovial fluid crystal analysis. Serum urate levels may be increased in patients with psoriatic arthritis, adding to the confusion. To aid dermatologists and general physicians in identifying those with psoriatic arthritis, a number of screening tools for arthritis in patients with psoriasis have been proposed and are the subject of a current head-to-head comparative study.
Laboratory Features
No diagnostic laboratory test for psoriatic arthritis is known. Although the absence of rheumatoid factor is considered an important distinguishing feature from RA, low levels of rheumatoid factor may be found in patients (5% to 16%) with typical psoriatic arthritis features. Until a more definitive diagnostic test becomes available, it is difficult to be categorical about diagnosis in these patients. Cyclic citrullinated peptide antibodies initially were thought to be specific to RA, but it is now recognized that cyclic citrullinated peptide antibodies are found in approximately 5% of psoriatic arthritis patients as well.27 Acute phase markers, such as erythrocyte sedimentation rate, C-reactive protein, and serum amyloid A, may be elevated in psoriatic arthritis patients, but less commonly and to a lesser degree than in RA patients. These markers are elevated in particular in patients with polyarticular disease and act as a marker of poor prognosis.28 Finally, as was mentioned previously, hyperuricemia may be found in association with metabolic abnormalities in psoriatic arthritis patients and may not reflect the extent of skin involvement.
Radiographic Features
Plain Radiography
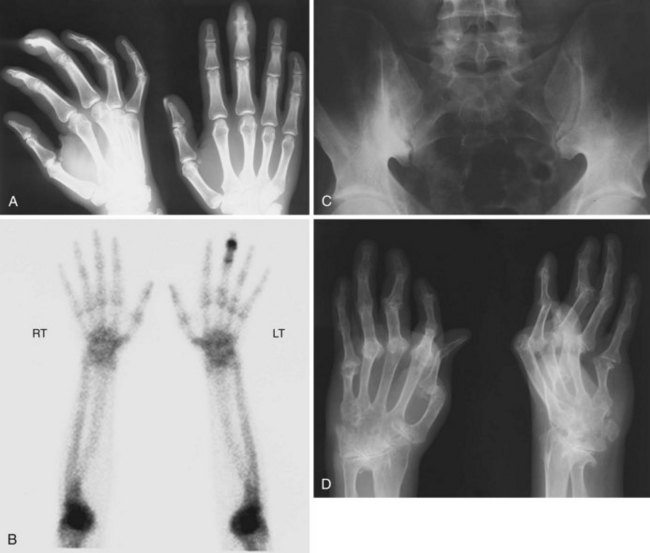
Sixty-seven percent of patients with established psoriatic arthritis have radiographic abnormalities,11 and 47% of patients with recent-onset psoriatic arthritis will have developed erosions within 2 years of disease onset.16 Distinctive radiographic features reflect in some cases the clinical phenotype (Figure 77-5). These features include asymmetric joint involvement; involvement of the interphalangeal joints of the fingers and toes, with features of bony erosion and resorption sometimes seen together and resulting in the classic “pencil-in-cup” deformity; joint space narrowing or involvement of entheseal sites, often with bony spurs or periostitis developing; and spinal involvement, frequently less severe and asymmetric than in classic ankylosing spondylitis.
Radiographic progression in psoriatic arthritis is slow in early stages of the disorder, with the mean modified Sharp (to include DIP joints in the hands) erosion score at presentation increasing from 1.2 to 3 at 2 years.16 Larson and Sharp scoring systems have been used in psoriatic arthritis, but neither the Larson nor the Sharp score has been developed specifically for psoriatic arthritis or has been extensively validated.
Musculoskeletal Ultrasound
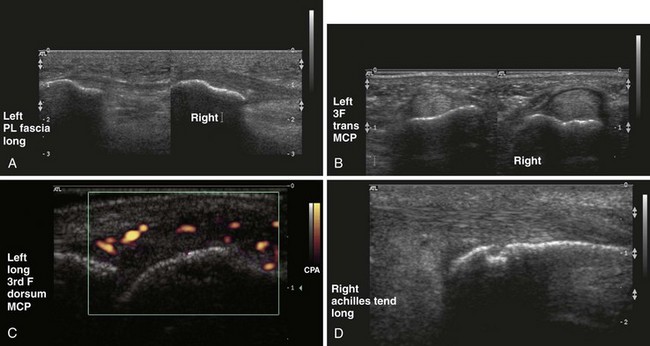
Many MSUS applications are useful in psoriatic arthritis; these applications are likely to develop further as the technology (in particular, power Doppler) that can be used to allow identification of blood flow is further advanced (Figure 77-6). Already it has been shown that MSUS is more sensitive than clinical examination in detecting knee synovitis in patients with various arthritides, including psoriatic arthritis.29 One study has suggested further that this increased sensitivity may result in reclassification of some patients as polyarticular when they were previously diagnosed as oligoarticular on clinical grounds.30 This reclassification may result in significant changes in prognosis and therapy. Finally, MSUS has been used in objective monitoring of the response of synovitis to therapy.31
MSUS features at the enthesis include entheseal thickening, hypoechoic change, increased vascularity as shown on power Doppler, tenosynovitis, and bony erosions or enthesophyte formation.32,33 MSUS has been shown to be more sensitive than clinical examination in detecting lower limb enthesopathy.33,34 MSUS has been used in studies of dactylitic digits. Together with MRI, MSUS has shown dactylitis to be due to a combination of synovial and tenosynovial inflammation.21,22 Finally, MSUS guidance for small joint or entheseal aspiration or injection may have particular application in patients with psoriatic arthritis.
Magnetic Resonance Imaging
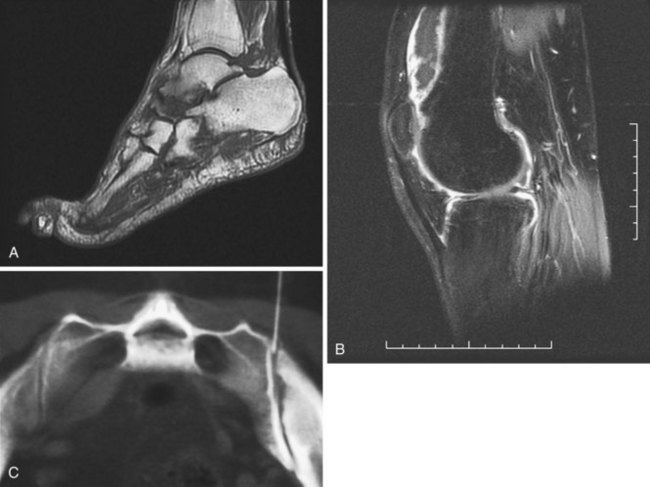
MRI studies have been particularly useful in offering new insights into disease pathogenesis in psoriatic arthritis. Based on the prominent entheseal-related bone marrow edema seen on MRI, McGonagle and colleagues35 have proposed that psoriatic arthritis, in contrast to RA, is an entheseal-based disease. MRI can be used to study all aspects of joint involvement, including the enthesis, but use of MRI as a routine clinical tool in psoriatic arthritis has not been clarified. Application of MRI to the spine or sacroiliac joints in psoriatic arthritis may prove especially helpful, as has been shown in ankylosing spondylitis, but studies in psoriatic arthritis patients are awaited. Preliminary studies have suggested that MRI can be useful as an outcome measure in the detection of synovitis or of vascularity in patients with psoriatic arthritis undergoing biologic therapies (Figure 77-7). More detailed studies are required. A new MRI scoring system has been proposed to measure articular inflammation and damage in patients with psoriatic arthritis (PsAMRIS).36
Other Imaging Modalities
The use of other imaging modalities, such as computed tomography (CT) or scintigraphy, has largely been superseded by MRI. CT is now reserved mainly for patients for whom MRI is contraindicated, or for whom MRI is unavailable. Positron emission tomography has been found to be comparable with MSUS and MRI in RA knees; this work needs to be extended to psoriatic arthritis. In a study using high-resolution CT imaging, structural bone changes were compared between RA and psoriatic arthritis. Smaller Ω-shaped and tubule-shaped erosions, as well as large sometimes corona-shaped osteophytes, are typical of psoriatic arthritis.37
Diagnosis
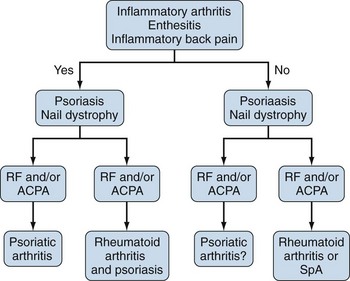
A diagnostic test for psoriatic arthritis is currently unavailable. Nevertheless, in its simplest form, psoriatic arthritis can be considered as an arthritis occurring in the presence of psoriasis, but in the absence of rheumatoid factor. Most psoriatic arthritis patients meet this simple definition. The arthritis can be predominantly spinal, it may involve only entheseal sites, psoriasis may present after arthritis in 15% of cases, and low-titer positive rheumatoid factor may be found. Recognizing these difficulties, the CASPAR group published new classification criteria based on an analysis of 588 psoriatic arthritis cases and 536 controls (Table 77-2).38 These criteria have yet to be validated in other large patient cohorts, and they should not be used in individual patient diagnosis. In the setting of clinical research, the CASPAR criteria have a specificity of 0.987 and a sensitivity of 0.914. For the individual patient, an algorithm for diagnosis is suggested in Figure 77-8.
Table 77-2 CASPAR Classification Criteria for Psoriatic Arthritis
Specificity 0.987, sensitivity 0.914.
CASPAR, Classification of Psoriatic Arthritis; ELISA, enzyme-linked immunosorbent assay.
* Current psoriasis scores 2, whereas all other items score 1.
Clinical Course and Outcome
Five early psoriatic arthritis cohorts have been studied.16,39–42 The mean disease duration in these cohorts was 6 to 12 months, the median age at onset of psoriasis was 27 to 31 years, and the median age at onset of arthritis was 38 to 52 years. Overall, little relationship was noted between skin disease severity and onset of psoriatic arthritis; the small joints of hands and feet were most commonly involved; the DIP joints were involved in one-third of patients, usually in association with nail disease, which was present in two-thirds; dactylitis and enthesitis were present in one-third; and spinal involvement only was found in 2% to 4%, but was present in 20% overall. At follow-up, disease had continued to progress in most patients, with 47% developing erosive disease within 2 years.16 Markers for progression included polyarticular disease and an elevated erythrocyte sedimentation rate. Long-term follow-up studies have shown significant morbidity and increased mortality in psoriatic arthritis: 17% of patients have five or more deformed joints, 40% to 57% have a deforming arthritis, 20% to 40% have spinal involvement, 11% to 19% are disabled, and mortality is increased compared with the general population.11,43,44 In one study, carotid intimal medial thickness was increased in psoriatic arthritis patients compared with controls (but was significantly reduced compared with an RA cohort of similar disease duration [unpublished observations]).
Comorbidities in Psoriatic Arthritis
Similar to the recent emphasis on the importance of comorbidities in psoriasis,45 a number of studies have highlighted cardiovascular risk factors and the metabolic syndrome in psoriatic arthritis. In one study of 109 psoriatic arthritis patients compared with 699 RA and 122 ankylosing spondylitis controls, the adjusted odds ratio for the metabolic syndrome was 2.44 (1.48 to 4.01; P < .001) with adjusted odds ratios significantly increased for central obesity, impaired fasting glucose, and low high-density lipoprotein (HDL) cholesterol.46 The European League Against Rheumatism (EULAR) has published evidence-based guidelines for cardiovascular risk management in patients with inflammatory arthritis.47
Outcome Domains and Instruments
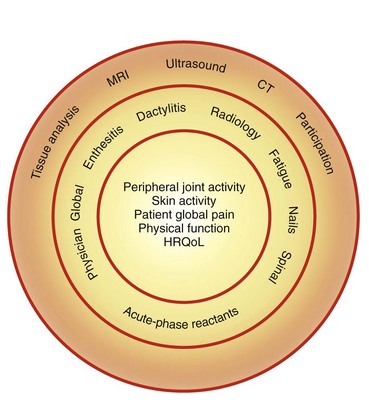
Measuring response to treatment of psoriatic arthritis in clinical trials has been the subject of much interest for members of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT). Many of the data that have been used to date in clinical trials have been adapted from RA and have not been validated. Controversial issues have included the number of joints to count, the usefulness of the acute phase response in psoriatic arthritis, and how important is it to include a measure of function or quality of life. An essential core set of domains that must be included in clinical trials has now been agreed on, with other domains necessary but not mandatory, and yet others requiring considerably more research (Figure 77-9). Instruments for many of these domains have yet to be developed and validated, and some instruments, such as the Psoriasis Assessment Severity Index (PASI), have acknowledged limitations. Table 77-3 lists the currently available instruments for the core domains. Instruments for dactylitis and enthesitis have been proposed and validated.48,49
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree