115 Poststreptococcal Arthritis and Rheumatic Fever
Although a dramatic decline in severity and mortality of the disease has been observed since the turn of the 20th century, reports in recent years have described its resurgence in the United States1 and in many military installations throughout the world—a reminder that the disease remains a public health problem even in developed countries. In addition, the disease continues essentially unabated in many developing countries. Estimates suggest that 10 to 20 million new cases will be reported per year in countries where two-thirds of the world population lives. For all of these reasons, it is important to keep rheumatic fever in the differential diagnosis of acute febrile illnesses, as well as acute inflammatory arthritis, in both children and adults.
Epidemiology
The incidence of ARF began to decline long before the introduction of antibiotics into clinical practice, decreasing from 250 to 100 patients per 100,000 population from 1862 to 1962 in Denmark.2 The introduction of antibiotics in 1950 rapidly accelerated this decline, until by 1980, the incidence ranged from 0.23 to 1.88 patients per 100,000, with disease occurring primarily in children and teenagers. A notable exception has been in the native Hawaiian and Maori populations (both of Polynesian ancestry), among whom the incidence continues to be 13.4 per 100,000 hospitalized children per year.3
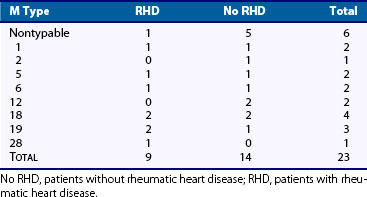
Only a few M serotypes (types 5, 14, 18, and 24) have been identified with outbreaks of ARF, suggesting that certain strains of group A streptococci may be more “rheumatogenic” than others.4 In Trinidad, types 41 and 11 have been the most common strains isolated from the oropharynx of patients with ARF. In our own series, conducted over a 20-year period (Table 115-1), many different M serotypes were isolated, including six strains that could not be typed. Kaplan and colleagues5 isolated several M types from patients seen during an outbreak of ARF in Utah, and these strains were mucoid and nonmucoid in character. Whether or not certain strains are more “rheumatogenic” than others remains unresolved. What is true, however, is that a streptococcal strain capable of causing well-documented pharyngitis is generally capable of causing ARF, although some notable exceptions have been recorded.6
Pathogenesis
Although little evidence suggests the direct involvement of group A streptococci in the affected tissues of ARF patients, a large body of epidemiologic and immunologic evidence indirectly implicates group A streptococci in initiation of the disease process: (1) It is well known that outbreaks of ARF closely follow epidemics of streptococcal sore throat or scarlet fever6; (2) adequate treatment of documented streptococcal pharyngitis markedly reduces the incidence of subsequent ARF7; (3) appropriate antimicrobial prophylaxis prevents recurrence of disease in known patients with ARF8; and (4) if one tests the sera of ARF patients for three antistreptococcal antibodies (streptolysin O, hyaluronidase, and streptokinase), most ARF patients (whether or not they recall an antecedent streptococcal sore throat) are found to have elevated antibody titers to these antigens.9
A note of caution is necessary concerning documentation (clinical or microbiologic) of an antecedent streptococcal infection. The frequency of isolation of group A streptococci from the oropharynx is extremely low, even in populations with limited access to antibiotics. An age-related discrepancy in the clinical documentation of an antecedent sore throat has been noted. In older children and young adults, the recollection of a streptococcal sore throat approaches 70%; in younger children, this rate approaches only 20%.1 It is important to have a high index of suspicion of ARF in children or young adults presenting with signs of arthritis or carditis or both, even in the absence of a clinically documented sore throat.
Another intriguing, and as yet unexplained, observation has been the invariable association of ARF only with streptococcal pharyngitis. Although many outbreaks of impetigo have occurred, ARF almost never occurs after infection with these strains. In Trinidad, where impetigo and ARF are common infections, the strains colonizing the skin are different from those associated with ARF, and this does not influence the incidence of ARF.10 The explanation for these observations remains obscure.
Group A streptococci fall into two main classes based on differences in the C-repeat regions of the M protein.11 One class is associated with streptococcal pharyngeal infection, and the other (with some exceptions) is commonly associated with impetigo. The particular strain of streptococci may be crucial in initiating the disease process. The pharyngeal site of infection with its large repository of lymphoid tissue also may be important in initiation of the abnormal humoral response by host to antigens cross-reactive with target organs. Finally, although impetigo strains do colonize the pharynx, they do not seem to elicit as strong an immunologic response to the M protein moiety as do the pharyngeal strains.12,13 This may prove to be an important factor, especially in light of known cross-reactions between various streptococcal structures and mammalian proteins.
Group A Streptococci
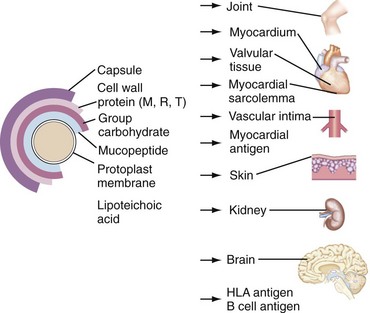
Figure 115-1 shows a schematic cross-section of group A streptococci. The capsule is composed of equimolar concentrations of N-acetyl glucosamine and glucuronic acid and is structurally identical to hyaluronic acid of mammalian tissues.14 Although numerous attempts to produce antibodies to this capsule have been unsuccessful,15,16 Fillet and colleagues17 were able to show high antibody titers to hyaluronic acid using techniques designed to detect nonprecipitating antibodies in the sera of immunized animals. Similar antibodies have been noted in humans.18 Data establishing the importance of this capsule in human infection have been almost nonexistent, although Stollerman19 commented on the presence of a large mucoid capsule as one of the more important characteristics of certain “rheumatogenic” strains.
With respect to the M protein moiety, investigations by Lancefield and others spanning almost 70 years20 have established that the M protein molecule (at least 80 distinct serologic types) is perhaps the most important virulence factor in group A streptococcal infection of humans. The protein is a helical, coiled-coil structure that bears a striking structural homology to the cardiac cytoskeletal proteins, tropomyosin and myosin, and to many other coiled-coil structures, including keratin, DNA, lamin, and vimentin. When the amino acid sequence of many M proteins was delineated, it was possible to localize specifically the cross-reactive areas of the molecules. Studies of Dale and Beachey21 showed that the segment of the M protein involved in the opsonic reaction cross-reacted with human sarcolemma antigens. Sargent and co-workers22 more precisely localized this cross-reaction to the M protein amino acid residues 164 through 197.
Evidence implicating these cross-reactions in the pathogenesis of ARF remains scant. Antibodies to myosin have been detected in the sera of ARF patients, but they also are present in a high percentage of the sera obtained from individuals who had a streptococcal infection but did not subsequently develop ARF.23 The significance of this observation is unclear because myosin is an internal protein of cardiac muscle cells and is not easily exposed to M protein cross-reacting antibodies. The group-specific carbohydrate of the streptococcus is a polysaccharide chain consisting of repeating units of rhamnose capped by N-acetyl glucosamine molecules. The N-acetyl glucosamine is immunodominant and gives rise to the serologic group specificity of group A streptococci.24
Goldstein and associates25 first described the cross-reaction between group A carbohydrate and valvular glycoproteins, and this reactivity was related to the N-acetyl glucosamine moiety present in both structures. Goldstein and Caravano26 noted that rheumatic fever (RF) sera reacted to the heart valve glycoprotein. Fillet (unpublished data) observed strong reactivity of RF sera with purified proteoglycan material. These cross-reactions could involve the sugar moiety present in the proteoglycan portion of the glycoprotein and the carbohydrate.
It generally has been assumed that group A anticarbohydrate antibodies do not play a role in phagocytosis of group A streptococci. Salvadori and co-workers27 showed, however, that human sera containing high titers of anti–group A carbohydrate antibody promoted opsonization and phagocytosis of many different M protein–specific strains, and the opsonophagocytic antibodies were directed to the N-acetyl glucosamine moiety of the group A carbohydrate. The mucopeptide portion of the cell wall is the “backbone” of the organism and is quite rigid in structure. It is composed of repeating units of muramic acid and N-acetyl glucosamine, cross-linked by peptide bridges.28 It is particularly difficult to degrade and induces a wide variety of lesions when injected into various species, including arthritis in rats29 and myocardial granulomas in mice resembling (but not identical to) RF Aschoff lesions.30
The relationship of cell wall mucopeptides to the pathogenesis of ARF remains obscure. Elevated levels of antimucopeptide antibody have been detected not only in the sera of patients with ARF, but also in the sera of patients with rheumatoid arthritis and juvenile rheumatoid arthritis31; however, its pathogenetic relationship to clinical disease has been difficult to establish. No evidence indicates that cell wall antigens are present in the Aschoff lesion or in the myocardial tissue obtained from patients with ARF. Perhaps the most significant cross-reactions lie in the streptococcal membrane structure. We have shown that immunization with membrane material32 elicited antibodies that were bound to heart sections in a pattern similar to that observed with acute RF sera (Figure 115-2).
Kingston and Glynn33 were the first to show that animals immunized with streptococcal antigens developed antibodies in their sera that stained astrocytes. Husby and associates34 showed that sera from ARF patients with chorea exhibited antibodies that were specific for caudate cells. Absorption of the sera with streptococcal membrane antigens eliminated reactivity with caudate cells. Numerous other cross-reactions between streptococcal membranes and other organs have been reported (e.g., renal basement membranes, basement membrane proteoglycans, skin [particularly keratin]). In the context of this chapter, space does not permit an exhaustive discussion of these cross-reactions, and the reader is referred to other studies35,36 for a more detailed discussion. Whether or not these cross-reactions (especially the cross-reactions seen with basement membranes and skin) play a role in the disease awaits further study.
Genetics
The concept that ARF might be the result of a host genetic predisposition has intrigued investigators for more than a century.37 It has been variously suggested that the disease gene is transmitted in an autosomal dominant fashion38 or in an autosomal recessive fashion with limited penetrance,39 or that it is possibly related to the genes conferring blood group secretor status.40 Renewed interest in the genetics of ARF occurred with recognition that gene products of the human major histocompatibility complex (MHC) were associated with certain clinical disease states. Using an alloserum from a multiparous donor, an increased frequency of a B cell alloantigen was reported in several genetically distinct and ethnically diverse populations of ARF patients and was not MHC related.41
More recently, a monoclonal antibody (D8/17) was prepared by immunizing mice with B cells from an ARF patient.42 A B cell antigen identified by this antibody was found to be expressed on increased numbers of B cells in 100% of rheumatic patients of diverse ethnic origins, and in only 10% of normal individuals. The antigen defined by this monoclonal antibody showed no association with or linkage to any of the known MHC haplotypes, and it did not seem to be related to B cell activation antigens. Studies with D8/17 have been expanded to a larger number of patients with RF (see Table 115-1) of diverse ethnic origins with essentially the same results. As discussed subsequently, the presence or absence of elevated levels of D8/17+ B cells in cases of questionable RF has been helpful in establishing or ruling out the diagnosis.
These studies contrast with other reports in which an increased frequency of HLA-DR4 and HLA-DR2 has been seen in white and black patients with rheumatic heart disease (RHD).43 Other studies have implicated HLA-DR1 and HLA-DRW6 as susceptibility factors in South African black patients with RHD.44 Guilherme and associates45 have reported an increased frequency of HLA-DR7 and HLA-DW53 in RF patients in Brazil.
These seemingly conflicting results concerning HLA antigens and RF susceptibility prompt speculation that these reported associations might be of class II genes close to (or in linkage disequilibrium with), but not identical to, the putative RF susceptibility gene. Alternatively, and more likely, susceptibility to ARF is polygenic, and the D8/17 antigen might be associated with only one of the genes (i.e., genes of the MHC complex encoding for DR antigens) conferring susceptibility. Although the explanation remains to be determined, the presence of the D8/17 antigen does seem to identify a population at special risk of contracting ARF (Table 115-2).
Table 115-2 Frequency of the D8/17 Marker in Patients with Rheumatic Fever, Patients with Other Diseases, and Controls in Various Geographic Populations
| Number | % Positive | |
|---|---|---|
| Rheumatic Fever Patients | ||
| New York | 43/45 | 93 |
| New Mexico | 30/31 | 97 |
| Utah* | 18/18 | 100 |
| Russia (Georgia) | 27/30 | 90 |
| Russia (Moscow) | 50/52 | 96 |
| Mexico | 35/39 | 89 |
| Chile | 45/50 | 90 |
| Normals | ||
| Russia | 4/78 | 5 |
| New York | 6/68 | 8 |
| Chile | 8/50 | 16 |
| Mexico | 6/72 | 8 |
| Other Diseases | ||
| Rheumatoid arthritis | 2/42 | 4 |
| Ischemic heart disease | 0/10 | 0 |
| Multiple sclerosis | 1/25 | 4 |
| Systemic lupus erythematosus | 1/12 | 9 |
Etiologic Considerations
Although a large body of immunologic and epidemiologic evidence has implicated group A streptococci in the induction of the disease process, the precise pathologic mechanisms involved remain obscure. At least three main theories have been proposed. The first theory is concerned with the question of whether persistence of the organism is important. Despite several controversial reports, no investigators have been able to show consistently and reproducibly live organisms in RF cardiac tissues or valves.46
The second theory revolves around the question of whether deposition of toxic products is required. Although an attractive hypothesis, little or no experimental evidence has been obtained to support this concept. Halbert and colleagues47 have suggested that streptolysin O (an extracellular product of group A streptococci) is cardiotoxic and might be carried to the site by circulating complexes containing streptolysin O and antibody. Despite an intensive search for these products, no such complexes in situ have been identified, however.48,49 Renewed interest in these extracellular toxins has emerged more recently with the observation by Schlievert and co-workers50 that certain streptococcal pyrogenic toxins (A and C) may act as superantigens. These antigens may stimulate large numbers of T cells through their unique bridging interaction with T cell receptors of specific Vβ types and class II MHC molecules. This interaction is distinct from conventional antigen presentation in the context of the MHC complex. When activated, these cells elaborate tumor necrosis factor, interferon-γ, and numerous interleukin moieties, contributing to the initiation of pathologic damage. It has been suggested51 that in certain disease states, such as rheumatoid arthritis, autoreactive cells of specific Vβ lineage may “home” to the target organ.
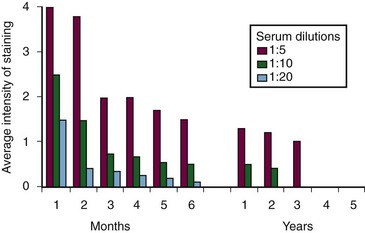
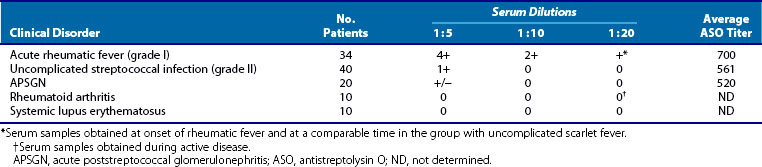
1. Employing a wide variety of methods, numerous investigators have documented the presence of heart-reactive antibodies in ARF sera. The prevalence of these antibodies has ranged from 33% to 85% in various series. Although these antibodies are seen in other individuals (notably individuals with uncomplicated streptococcal infections that do not progress to RF and patients with poststreptococcal glomerulonephritis), the titers are always lower than those seen in RF and decrease with time during the convalescent period (Table 115-3). An important point in terms of diagnosis and prognosis has been the observation by Zabriskie and associates52 that these heart-reactive antibody titers decline over time. By the end of 3 years, these titers are essentially undetectable in patients who had only a single attack (Figure 115-3). This pattern is consistent with the well-known clinical observation that recurrences of RF most often occur within the first 2 to 3 years after the initial attack and become rarer 5 years after an initial episode.
Table 115-3 Heart-Reactive Antibody Titers in Sera of Patients with Acute Rheumatic Fever Compared with Uncomplicated Streptococcal Infections
As illustrated in Figure 115-4, this pattern of titers also has prognostic value. During the 2- to 5-year period after the initial attack, a patient’s titers decreased to undetectable levels. With a known break in prophylaxis starting in year 6, at least two streptococcal infections occurred, as evidenced by an increase in antistreptolysin O (ASO) titers during that period. The concomitant increase in heart-reactive antibody titers was notable. The final infection was followed by a clinical recurrence of classic rheumatic carditis complete with isolation of the organism, elevated heart-reactive antibodies, and acute phase reactants 11 years after the initial attack.
2. Sera from patients with ARF also contain increased levels of antibodies to myosin and tropomyosin compared with sera from patients with pharyngeal streptococcal infections that do not progress to ARF. These myosin affinity purified antibodies also cross-react with M protein moieties, suggesting that this molecule could be the antigenic stimulus for the production of myosin antibodies in these sera.23,53
3. Finally, as indicated earlier, autoimmune antibodies are a prominent finding in chorea, another major clinical manifestation of ARF, and these antibodies are directed against the cells of the caudate nucleus. The titer of this antibody corresponds with clinical disease activity.34 Although not autoimmune in nature, the presence of elevated levels of immune complexes in ARF has been well documented in the sera and in the joints of ARF patients.54 Elevated levels of immune complexes, which may be as high as the levels seen in classic poststreptococcal glomerulonephritis, may be responsible for the immune complex vasculitis seen in ARF tissues and may provide the initial impetus for vascular damage, followed by the secondary penetration of autoreactive antibodies. Support for this concept is the close clinical similarity of RF arthritis to experimentally induced serum sickness in animals or the arthritis seen secondary to drug hypersensitivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree