Other bone diseases in the elderly
Complex regional pain syndrome
INTRODUCTION
Osteoporosis is by far the most important cause of fragility fractures in elderly people, but causes of musculoskeletal pain, deformity and fractures include metastatic bone disease, Paget’s disease of bone (PDB) and osteomalacia. The reader is referred to Chapter 16 for a discussion of metastatic bone disease. Here the clinical presentation, differential diagnosis and treatment of PDB, osteomalacia, complex regional pain syndrome (CRPS), primary hyperparathyroidism (PHPT) and renal bone disease are discussed.
PAGET’S DISEASE OF BONE
PDB is a common metabolic bone disease in people of European descent. At a cellular level it is characterized by focal areas of increased and disorganized bone remodelling which can affect one or several bones in the skeleton.1 Paget’s disease preferentially affects the axial skeleton, and the bones that are most commonly affected include the pelvis, femur, tibia, lumbar spine, skull and scapula.2 Paget’s disease is often asymptomatic and reflecting this fact, it has been estimated that only 10–20% of patients come to medical attention.3 Bone pain and other complications of the disease are common in those who do present clinically however, leading to significant morbidity in some patients.
Epidemiology
Paget’s disease is rare before the age of 40, but gradually increases in incidence thereafter to affect up to 8% of the UK population by the age of 85. Paget’s disease is common in Caucasians from north-west and southern Europe but is rare in Scandinavians, Asians, Chinese and Japanese. These ethnic differences persist after migration, supporting the importance of genetic factors in the aetiology. Nonetheless, the incidence of PDB has fallen in most countries over the past 25 years, suggesting that environmental factors also play a role. Suggested environmental triggers for PDB include paramyxovirus infections,4 dietary calcium deficiency during childhood,5 vitamin D deficiency,6 repetitive mechanical loading or skeletal injury7 and exposure to environmental toxins.8 Paramyxovirus infection is the only factor that has been investigated experimentally, but the results of studies that have attempted to isolate viral proteins and nucleic acids from Pagetic tissue have been conflicting.4,9
Pathophysiology
The primary abnormality is increased osteoclastic bone resorption. The osteoclasts in PDB are larger than normal and some contain nuclear inclusion bodies (Figure 11.1). These were previously thought to represent paramyxovirus nucleocapsids,10 but there is increasing evidence to suggest that they may be abnormal protein aggregates due to defects of the autophagy pathway.10 Paget’s disease is also associated with increased bone formation, marrow fibrosis and increased vascularity of bone. Although the density of affected bone is often increased due to osteosclerosis, the bone that is formed is structurally abnormal since the bone lamellae are laid down in a chaotic fashion (woven bone), reducing mechanical strength.
Over the past 10 years, it has become clear that genetic factors are important in the pathogenesis of PDB.11 Many patients have a positive family history of the disease and the risk of PDB developing in a first degree relative of an affected patient is sevenfold increased as compared with an unrelated individual.12 The disease can also be inherited in an autosomal dominant manner and this pattern is observed in between 15% and 40% of families.13,14
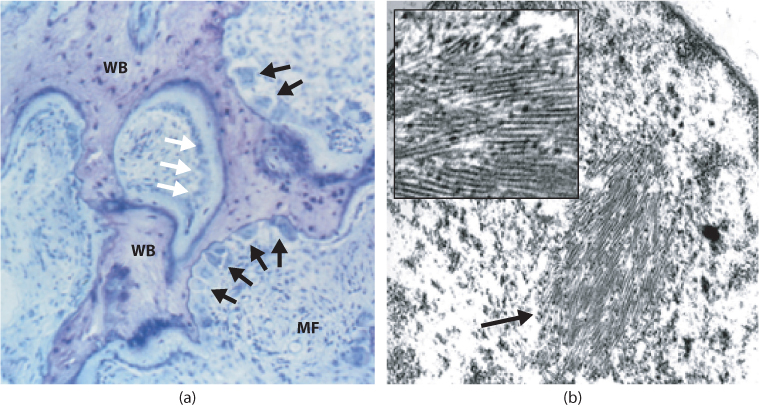
Figure 11.1 Histological features of PDB. (a) Toluidine blue stained transiliac bone biopsy in a patient with active Paget’s disease. The calcified bone stains dark blue and the osteoid stains light blue. There are typical features of active PDB with a marked increase in osteoclast numbers and eroded surfaces (black arrows) juxtaposed with increased bone formation (white arrows). There is extensive marrow fibrosis (MF) and woven bone (WB). (b) Typical nuclear inclusion body in a Pagetic osteoclast visualized by transmission electron microscopy. The inset shows a higher power view of the same lesion.
Linkage analysis in families coupled with genome wide association studies (GWAS) have identified several genes and loci that predispose to PDB.15,16,17 and 18 These are summarized in Table 11.1. Most implicated genes are known to play a role in osteoclast differentiation or function. The most important PDB-susceptibility gene is SQSTM1 which lies on chromosome 5q35 and encodes the p62 protein. This protein acts as a scaffold in the receptor activator of the nuclear factor kappa B (RANK) signalling pathway which plays a key role in osteoclast differentiation and activity. The disease associated mutations in PDB cluster in the ubiquitin associated (UBA) domain of the protein and through various mechanisms cause activation of RANK signalling and stimulate osteoclastogenesis.19,20 The TNFRSF11A gene which encodes the receptor activator of RANK is mutated in the rare PDB-like syndromes familial expansile osteolysis (FEO), expansile skeletal hyperphosphatasia (ESH) and early-onset familial Paget’s disease (EoPDB). All of these conditions present with an early-onset severe PDB-like phenotype during adolescence. The mutations cluster in the signal peptide and cause osteoclast activation.11 In addition, GWAS in classical PDB have identified an association between common variants at the TNFRSF11A locus and classical PDB. The molecular mechanisms responsible are incompletely understood. Loss of function mutations in the TNFRSF11B gene which encodes osteoprotegerin (OPG) cause juvenile PDB which presents with a severe phenotype during childhood. Mutations in the VCP, hnRNPA2B1 and hnRNPA1 genes cause the rare syndrome of inclusion body myopathy, Paget’s disease and frontotemporal dementia. The causal mutations result in accumulation of abnormal protein aggregates in brain, muscle and bone.
The OPTN gene on chromosome 10p13 has been implicated as a cause of familial PDB in patients who do not have SQSTM1 mutations by linkage analysis21 and as a cause of non-familial PDB by genome wide association analysis.18 The predisposing variant is associated with reduced mRNA expression of OPTN. This suggests that OPTN is an inhibitor of osteoclast differentiation and/or activity and that the reduced levels of expression predispose to PDB by causing osteoclast activation. At the present time the disease-causing mutations at the OPTN locus have not been identified. GWAS have identified other loci that predispose to PDB within or close to the CSF1, RIN3, DCSTAMP, NUP205 and PML genes.17,18 The CSF1 gene encodes macrophage colony stimulating factor which plays an essential role in osteoclast differentiation22; the DCSTAMP gene encodes dendrocyte expressed seven transmembrane protein which plays an essential role in promoting the fusion of mononuclear osteoclast precursors to multinucleated osteoclasts.23 At the present time the role of the RIN3, NUP205 and PML genes in bone metabolism are unclear, but it seems likely that these genes (or genes nearby) will turn out to play a role in osteoclast differentiation or function.
Clinical features
Paget’s disease can present in a variety of ways but the most common is with bone pain, which is the presenting feature in 50–70% of cases. Other modes of presentation include bone deformity, deafness and pathological fractures. In a recent case series from the United Kingdom, pain was a presenting feature in 50%, deformity in 18%, deafness in 6% and pathological fracture in 5%.24 In the same series, about 20% of patients were truly asymptomatic and the disease was picked up as an incidental finding in patients who had blood tests or X-rays for other reasons.24
Table 11.1 Genes and loci that predispose to Paget’s disease of bone
Clinical signs of PDB include bone deformity and expansion, increased warmth over affected bones, and pathological fracture. Bone deformity is most evident in weight-bearing bones such as the femur and tibia (Figure 11.2), but when the skull is affected the patient may complain that hats no longer fit properly due to cranial enlargement. Neurological problems, such as deafness, cranial nerve defects, nerve root pain, spinal cord compression and spinal stenosis, are recognized complications due to enlargement of affected bones and encroachment upon the spinal cord or cranial nerve foramina. Surprisingly, deafness seldom results from compression of the auditory nerve, but is instead conductive in nature due to osteosclerosis of the temporal bone.25,26 The increased vascularity of Pagetic bone has been reported to precipitate high-output cardiac failure in elderly patients with limited cardiac reserve, but this is extremely rare. Hypercalcaemia may occur in patients who are immobilized. Osteosarcoma is a rare but serious complication affecting less than 0.01% of cases. The presentation is with worsening pain and/or swelling of an affected site.
Investigations
The diagnosis of PDB can usually be made by X-ray, which shows the typical features of bone expansion with an abnormal trabecular pattern, cortical thickening and alternating areas of radiolucency and osteosclerosis (Figure 11.2). The serum levels of alkaline phosphatase (ALP) are typically raised but may be normal, either because the disease is limited to a single bone, or because the disease is metabolically inactive. Routine biochemical tests in PDB are otherwise normal, resulting in a pattern of an ‘isolated’ elevation in ALP which is typical of the disease. Radionuclide bone scanning is a useful way of defining the presence and extent of PDB since tracer is avidly taken up by sites in which bone turnover is increased. This gives a picture of intense contiguous tracer uptake in an affected bone which is virtually diagnostic (Figure 11.2). Paget’s disease can occasionally be confused with osteosclerotic metastases. Although the radiographic and bone scan appearances usually allow the correct diagnosis to be made, bone biopsy of an affected site can sometimes be required. In this regard it should be noted that PDB and bone metastases can co-exist.
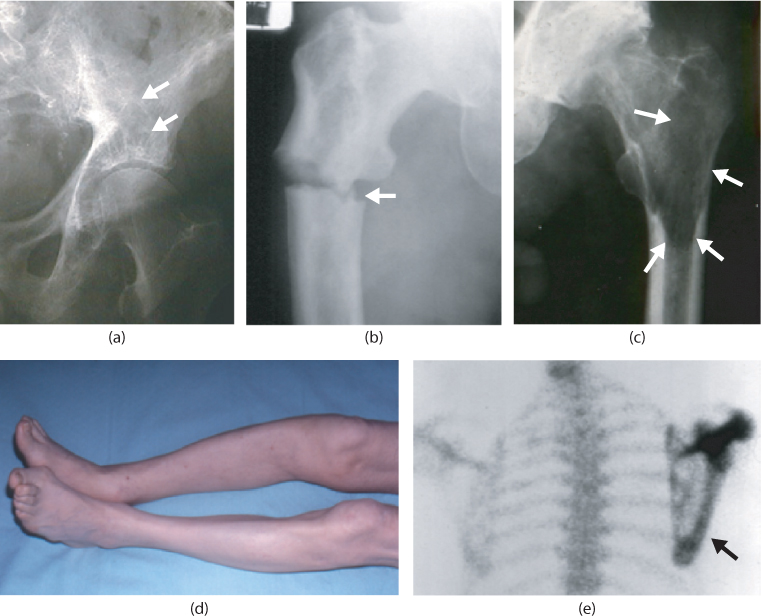
Figure 11.2 Clinical and radiographic features of PDB. (a) Radiograph of a patient with PDB of the left hemipelvis showing abnormal trabecular architecture with alternating areas of osteosclerosis and osteolysis (white arrows). (b) Pathological fracture (white arrow) in a patient with Paget’s disease of the right femur. (c) Osteolytic lesion of the femoral shaft of a patient with Paget’s disease of the left femur. The lytic area is highlighted by white arrows. There is also osteosclerosis of the femoral head and secondary osteoarthritis of the hip joint. (d) Tibial deformity in a patient with PDB. (e) Radionuclide bone scan of a patient with Paget’s disease of the scapula showing intense homogeneous tracer uptake of the affected bone.
Monitoring levels of metabolic activity in PDB is most easily and conveniently done by measurements of ALP which are raised in patients with high bone turnover and lowered by bisphosphonate therapy and other antiresorptive agents used in the treatment of PDB. Although ALP levels give an indication of the levels of bone turnover in PDB, they do not correlate well with symptoms such as bone pain1 and have not as yet been shown to act as a predictor of complications such as fracture or deformity.
Medical management
The main aim of medical management is to improve bone pain and this can be addressed by antiresorptive therapy or by the use of analgesics. There is no evidence as yet to show that medical treatment can prevent or reverse complications of PDB such as fracture, deformity, deafness and osteoarthritis.2
The increased bone turnover in PDB can be reduced by therapy with osteoclast inhibitors. Although various osteoclast inhibitors have been used in PDB, the current treatments of choice are bisphosphonates. Bisphosphonates are a class of compounds related to pyrophosphate in which phosphonate groups are linked by a carbon atom to which various side chains can be attached.27 The phosphonate moiety binds calcium and causes the bisphosphonates to bind avidly to hydroxyapatite crystals in bone. Bisphosphonates particularly target to areas of increased bone turnover when they are taken up by osteoclasts that resorb bone. The bisphosphonate is then released within the osteoclast, impairing cellular function and causing cell death, making them ideal candidates for the treatment of PDB.1
The main indication for treatment with bisphosphonates in PDB is bone pain localized to an affected site which is thought to be due to increased metabolic activity.1 Although bisphosphonates are effective at treating bone pain secondary to active PDB, it is sometimes difficult to differentiate this type of pain from that caused by a co-existing condition such as osteoarthritis or nerve compression. Therefore careful clinical evaluation is required to determine the likely cause of the pain in patients with PDB. This is not always easy; indeed in a recent case series from a specialist clinic, about one third of patients thought to have pain due to increased metabolic activity did not respond to bisphosphonate treatment, indicating that another cause may have been responsible.
Several bisphosphonates have been licensed for the treatment of PDB but the most widely used are the nitrogen containing bisphosphonates pamidronate, zoledronate and risedronate (Table 11.2). These are more effective than simple bisphosphonates such as etidronate and tiludronate at suppressing bone turnover in PDB. Zoledronic acid in particular is highly effective at restoring levels of bone turnover to normal in PDB with effects that last for several years after a single infusion.33 It should be noted that the effectiveness with which bisphosphonates suppress bone turnover correlates poorly with the response of pain. This is most probably due to the fact that pain can arise from causes other than increased metabolic activity and that it may not be necessary to completely normalize bone turnover to get a positive response on pain.2 Calcitonin is very occasionally used in the treatment of PDB where bisphosphonates are contraindicated. However this is less convenient to administer than bisphosphonates, is much more expensive and has a short duration of action. Repeated courses of antiresorptive drugs can be given if symptoms recur, particularly if this is accompanied by evidence of increased bone turnover.
Bone pain in PDB can also be treated with analgesics, non-steroidal anti-inflammatory drugs (NSAID) and anti-neuropathic agents. Although these agents have not specifically been studied in clinical trials of bone pain in PDB, clinical experience indicates that they are often effective. Indeed, in the Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) trial, therapy with analgesics and other painkillers coupled with intermittent bisphosphonate therapy in patients who did not respond adequately was found to be equivalent to intensive bisphosphonate therapy in the treatment of Pagetic bone pain.2
Surgical treatment
There are several indications for surgical treatment of PDB (Table 11.3), but the three most common are pathological fractures, osteoarthritis and spinal stenosis.
In general terms, surgical treatment of PDB can be challenging due to the increased vascularity of bone, bone deformity and osteosclerosis, but despite this the results of surgical treatment for PDB are generally good.34 In particular, fracture healing in PDB seems to proceed normally with no real evidence to suggest an increased risk of non-union or delayed union.35
It has been traditionally considered that patients with PDB should be treated with osteoclast inhibitors to try and normalize bone turnover before surgery goes ahead, but there is no good evidence to suggest that this makes any difference. Indeed the only study to look at this, which was only published in abstract form, showed no evidence of a difference in blood loss in PDB patients treated with bisphosphonates as opposed to those who were untreated.
Orthopaedic surgery may also be required in patients who develop osteosarcoma, but the prognosis is poor even with aggressive operative treatment with an overall 5-year survival of about 6%.36
Table 11.2 Bisphosphonates used in the treatment of Paget’s disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








