11 Neutrophils
Neutrophils
Neutrophil Myelopoiesis and Clearance
The neutrophil majority in the bloodstream is duplicated in the bone marrow, where 60% of hematopoietic capacity may be dedicated to neutrophil production. Daily, 1011 neutrophils are released into the bloodstream. Neutrophil development in the marrow takes about 14 days, originating from the hematopoietic stem cell. Stem cells fated to become neutrophils first differentiate into myeloblasts, which retain the capacity to develop into eosinophils, basophils, and neutrophils. Subsequent differentiation leads to the neutrophilic promyelocyte, a dedicated precursor of the neutrophil, and proceeds through the stages of neutrophilic myelocyte, metamyelocyte, band cell, and mature neutrophil. At the metamyelocyte stage, neutrophil mitosis ceases, whereas neutrophil development and organization of granules continue. Neutrophils are terminally differentiated; they neither divide nor alter their gross phenotype after their release from the marrow.
Given the origin of neutrophils in a pluripotent stem cell, as well as the precise phases of their development, the mechanisms regulating neutrophil differentiation are of considerable interest. Although incompletely understood, studies have emphasized the role of a particular complement of transcription factors and cytokines that seem to direct the early cells toward neutrophil development. Several myeloid factors are necessary for the transcriptional regulation of neutrophils including LEF-1, CCAAT enhancer binding proteins α and ε (C/EBPα and C/EBPε), and GFI-1. Principal among the cytokines regulating granulopoiesis is granulocyte colony-stimulating factor (G-CSF). G-CSF effects include induction of myeloid differentiation, proliferation of granulocyte precursors, and release of mature neutrophils from the marrow.1 Biologic effects of G-CSF are mediated through its receptor (G-CSFR or CD114), a member of the class I cytokine receptor family. Although other hematopoietic cytokines contribute to granulopoiesis in vivo (including granulocyte-macrophage colony-stimulating factor [GM-CSF], interleukin [IL]-6 and IL-3), their individual presence is not essential as demonstrated by knockout experiments.
Once mature, neutrophils exit the bone marrow through the tight-fitting pores of the sinusoidal endothelium and enter the circulation, a process called transcellular migration.2 Neutrophils released from the marrow have a bloodstream half-life of approximately 6 hours and a tissue half-life only marginally longer. Neutrophil life spans may be modulated by soluble signals: when exposed to stimuli such as tumor necrosis factor (TNF) and Fas (CD95) ligand, neutrophils undergo apoptosis or programmed cell death.3,4 The high output and short half-life of neutrophils imply that neutrophil clearance mechanisms must exist. Recently, the SDF-1/CXCR4 signaling system has been implicated in neutrophil clearance. CXC chemokine receptor 4 (CXCR4), a G-protein coupled receptor, is expressed at low levels in the mature neutrophil. As they age, neutrophils alter their phenotype and upregulate CXCR4. This change supports homing to the bone marrow via the chemoattractant stromal-derived factor 1 (SDF-1 or CXCL12). Once back in the marrow, senescent neutrophils are phagocytosed by stromal macrophages.5 Senescent or apoptotic bloodstream neutrophils are also cleared by liver and spleen macrophages (reticuloendothelial system). Although little is known about the molecular mechanisms underlying neutrophil clearance in the liver and spleen, upregulation of the adhesion molecule P-selectin on Kupffer cells appears to be relevant. It remains a matter of speculation whether tissue neutrophils are cleared primarily via local macrophages.
Neutrophil Morphology and Content
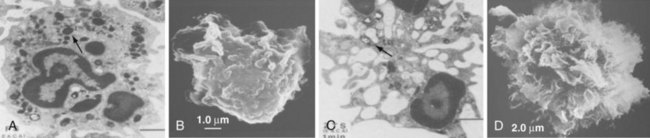
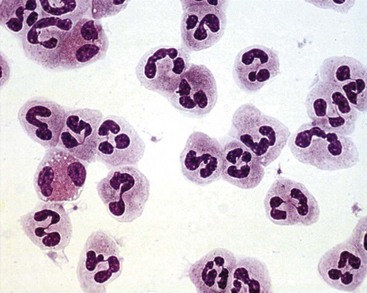
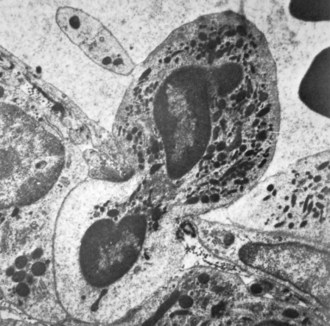
Neutrophil nuclei tend to have more lobes than nuclei of other polymorphs, typically three to five (Figures 11-1 and 11-2). The multilobed nature of this nucleus reflects a condensation of chromatin and suggests that neutrophils might be incapable of transcription. It is now appreciated, however, that neutrophils retain the capacity for constitutive and stimulated protein synthesis, albeit at a limited rate.
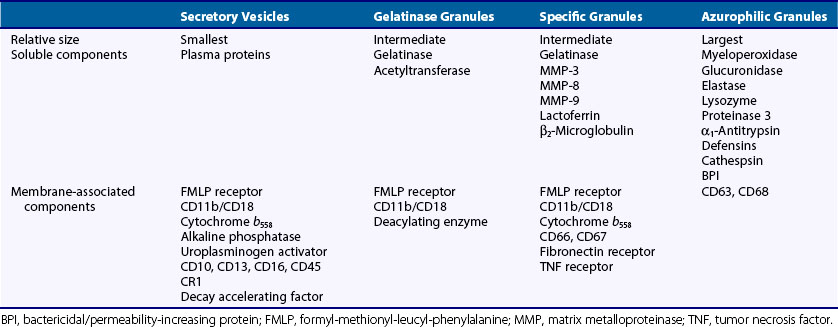
Neutrophil granules identifiable by classic histochemical staining comprise two classes (see Figure 11-1). Neutrophil primary granules form first and, by virtue of their staining tendencies (affinity for the basic dye azure A), are also referred to as azurophilic granules.6 These granules are oval or round and vary in size. They are similar, and functionally equivalent, to the lysosomes of other cells. In contrast, neutrophil secondary granules constitute a population unique to neutrophils, a fact reflected in the alternative nomenclature of specific granules often used to describe these structures. Characteristic of azurophilic granules is the presence of myeloperoxidase (MPO), an enzyme that catalyzes the formation of hypochlorous acid from chloride in the presence of superoxide anion (O2−). The presence of large amounts of this enzyme in azurophilic granules lends collections of neutrophils (pus) their typical greenish yellow color. Consistent with their role as lysosomes, azurophilic granules also contain a variety of proteases and other enzymes including elastase, lysozyme, acid phosphatase, and cathepsins and enzymes directed at nucleic acids and sugars. At the membrane level, however, they differ from true lysosomes because they lack lysosome-associated membrane proteins 1 and 2 (LAMP-1 and LAMP-2) and the mannose-6-phosphate receptor system.7 In contrast to azurophilic granules, specific granules possess an extensive array of membrane-associated proteins including cytochromes, signaling molecules, and receptors. Specific granules constitute a reservoir of proteins destined for topologically external surfaces of phagocytic vacuoles and the plasma membrane (Table 11-1).6 One particularly important family of proteinases found in neutrophil specific granules are the matrix metalloproteinases (MMPs) including neutrophil collagenase-2 (MMP-8), gelatinase-B (MMP-9), stromelysin (MMP-3), and leukolysin (MMP-25). They are stored as inactive proenzymes and undergo proteolytic activation after granule fusion and interaction with azurophilic granules, gaining the ability to alter integral components of bacterial membranes. Neutrophil MMP function is not limited to bacterial killing because MMPs are also important for extravasation and diapedesis.8
Further study has confirmed the existence of two additional classes of vesicles. Gelatinase granules are almost identical in size to specific granules and share some proteins in common with them. As their name implies, however, gelatinase granules are distinguished by their high concentrations of gelatinase, a latent enzyme with the capacity for tissue destruction.9 Secretory vesicles are smaller and lighter than the other classes and do not seem to contain proteolytic enzymes.10 Rather, secretory vesicles are noteworthy for an extensive complement of membrane-associated proteins including receptors otherwise identified with the plasma membrane. These and other data suggest that the secretory vesicle is a reservoir of neutrophil plasma membrane and other membrane proteins.
Azurophilic and specific granules further contain antimicrobial proteins and peptides that are a cornerstone of innate immunity. A detailed description of the neutrophil’s armamentarium against foreign invaders is beyond the scope of this chapter, but a few whose mechanisms of action have more recently been elucidated warrant mention. Elastase, mentioned previously, aids in the killing of gram-negative bacteria via degradation of bacterial outer membrane protein A.11 Elastase-deficient mice are more susceptible to infection with gram-negative (but not gram-positive) organisms than wild-type mice. The defensins, normally located in azurophilic granules, are found in mg/mL concentrations in the phagocytic vacuole (see later) and render target cell membranes permeable. Bactericidal/permeability-inducing protein, also located in azurophilic granules, acts in concert with the defensins; it potently neutralizes endotoxin and is cytotoxic to gram-negative bacteria. Bactericidal/permeability-inducing protein also enhances the activity of secretory phospholipase A2, which has activity against gram-negative and gram-positive bacteria. Lactoferrin (found in specific granules) deprives microorganisms of iron and has antiviral and antibacterial effects. Other granule-associated proteins such as cysteine-rich secretory protein 3 (CRISP3) and ficolin 1 have recently been described, although their function is still unclear.
Neutrophil granule contents might play important roles beyond their direct antimicrobial effects; they also may amplify or dampen the innate and adaptive immune response. Lactoferrin released during phagocytosis may inhibit proliferation of mixed lymphocyte cultures by decreasing release of interleukin (IL)-2, TNF, and IL-1. Proteinase 3 has been found to augment release of active TNF and IL-1 in monocyte/neutrophil co-cultures by releasing the membrane-bound forms of these cytokines.12 Gelatinase B has been shown to convert latent IL-1 into its active form13 and to potentiate IL-8 activity by truncating this chemoattractant and increasing its release, consequently amplifying neutrophilic influx.14 Neutrophil elastase also may play a proinflammatory role by virtue of its ability to cleave and disrupt phosphatidyl serine receptors on macrophages. Apoptotic cells undergo membrane alterations that lead to expression of phosphatidyl serine on their outer membrane surface, and interaction of phosphatidyl serine with its receptor leads to macrophage responses that downregulate inflammation through the generation of transforming growth factor (TGF)-β.15 By disrupting these interactions, the presence of neutrophil elastase may permit inflammation to continue.
Neutrophil Activation and Function
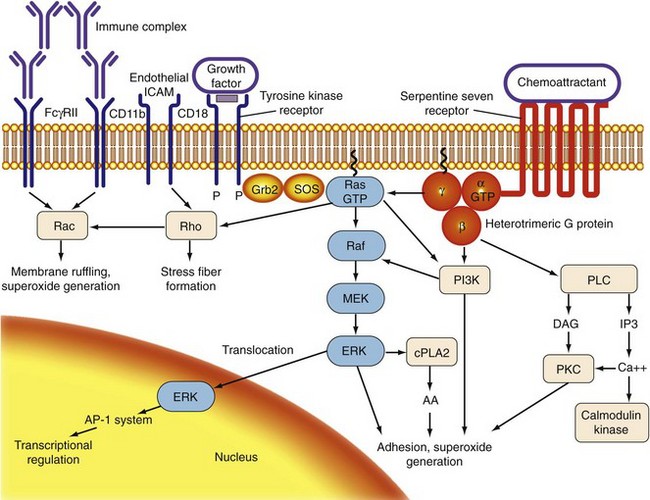
For bloodstream neutrophils to destroy foreign targets in the periphery, they first must sense the presence of such targets at a distance. They must then attach to the activated endothelium through multiple interactions involving adhesion molecules and their receptors (rolling and adhesion). After passing through the endothelium of postcapillary venules (diapedesis), neutrophils migrate to the source of the signal (chemotaxis). Finally, neutrophils must encounter a target, engulf it, and destroy it. Collectively, these processes are referred to as neutrophil activation. Because of the potential for tissue destruction, neutrophil activation must be carefully regulated. The internal responses through which a cell translates an encounter with a stimulus into a particular phenotypic response are termed signal transduction (Figure 11-3).16
Stimuli and Receptors
Classic chemoattractants include lipid mediators (e.g., leukotriene B4 [LTB4], platelet-activating factor) and proteins/peptides (e.g., formylated peptides, the complement split product C5a, and IL-8). Chemoattractants in vivo are formed at sites of inflammation—either produced at the site by inflammatory cells such as LTB4 or IL-8 or liberated from already synthesized proteins, as in the case of C5a. The ability of formylated peptides such as N-formyl-methionyl-leucyl-phenylalanine to stimulate neutrophils probably represents a particularly ancient arm of the innate immune response because prokaryotic, but not eukaryotic, cells synthesize proteins whose first amino acid is a formylated methionine. Chemoattractants also have the capacity to stimulate most other aspects of neutrophil activation. Their individual potencies for particular responses may differ,17 however, suggesting that they may serve overlapping but distinct functions in neutrophil activation. CXC chemokines are a recently described group of chemoattractants, characterized by the presence of two N-terminal cysteines (“C”) separated by any other amino acid (“X”) at the carboxy terminus. Many CXC chemokines appear essential for recruitment including IL-8 (CXCL8), KC (CXCL1), and MIP-2 (CXCL2).
Bloodstream activation of neutrophils depends on the presence of specific surface receptors. Most chemoattractant receptors belong to a class known as seven-transmembrane-domain receptors, “serpentine seven” receptors, or G protein-coupled receptors (GPCR); these receptors are composed of a single protein chain whose seven hydrophobic domains span the plasma membrane.18 Binding of a particular chemoattractant occurs in a pocket on the cytoplasmic face, close to or below the level of the plasma membrane. Receptors for soluble ligands other than chemoattractants have also been identified on neutrophils including receptors for growth factors, colony-stimulating factors, and cytokines, as described. These receptors fall into several families distinct from the serpentine sevens. Growth factor receptors are members of the protein tyrosine kinase receptor family, in which ligand interaction with two identical or related receptors brings them into proximity, causing their phosphorylation and activation. Recent systems biology studies have shown that receptors for a variety of inflammatory stimuli become highly expressed only in mature neutrophils.19 The most notable examples include CXC and CC chemokine receptors such as IL-8R-α and β; CXCR-4 and CCR-1, 2, and 3; the cytokine receptors for TNF 1 and 2; interferon (IFN)-α and -γ; and interleukin receptors IL1R, IL4R, IL6R, IL10R, and IL17R. Some nonchemoattractant ligands do not directly activate neutrophils but may modulate their function. Pretreatment of neutrophils with either insulin or GM-CSF results in amplification of subsequent neutrophil responses to chemoattractants, a process referred to as priming.
G Proteins
A monomeric class of low-molecular-weight (20 to 25 kD) GTP-binding proteins (LMW-GBPs) has also been described. Because the first prototypic LMW-GBP to be described was the proto-oncogene Ras, these also are referred to as Ras-related or Ras superfamily proteins, or small GTPases. LMW-GBPs combine, in one molecule, the prenyl and methyl modifications of the G protein γ subunit with the GTP-binding capacity of the α subunit. At least four families of LMW-GBP have been described: the Ras family, whose members play roles in activation as well as cell growth and division; the Rho family, which functions in cytoskeletal rearrangements; and the Rab and Arf families, which are crucial for vesicular and endomembrane trafficking.20 All four classes of LMW-GBPs are represented in neutrophils.
Second Messengers
Second messengers are small, diffusible molecules that are generated in response to stimuli and transmit signals from membrane receptors to downstream effector proteins. In the classic model of neutrophil activation, engagement of receptors results in the activation of phospholipase C, which cleaves phosphatidylinositol triphosphate (PIP3) into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). DAG and IP3 mediate the influx of cytosolic calcium and the activation of protein kinase C (PKC). Other phospholipases present in the neutrophil include phospholipase A2 (cPLA2,) which cleaves phosphatidylcholine or ethanolamine, or both, and is responsible for the generation of arachidonic acid (AA) and phospholipase D, which cleaves phosphatidylcholine into phosphatidic acid and choline.21 Although all the previously described second messengers have been implicated in neutrophil activation, other lipid mediators may have negative regulatory effects. Sphingosine and ceramide each inhibits neutrophil phagocytosis.
In addition to lipids, other organic and inorganic messenger molecules have been characterized. Intracellular concentrations of cyclic adenosine monophosphate (cAMP), a classic second messenger, increase rapidly in neutrophils exposed to stimuli and inhibitors. cAMP in these settings is likely to provide a negative regulatory (off) signal because direct exposure to cAMP inhibits neutrophil responses, probably through the activation of protein kinase A (PKA).17 In contrast, increases in cyclic guanosine monophosphate (cGMP) have a modest enhancing effect on some neutrophil responses. Nitric oxide (NO), an important molecule in the regulation of host defense, is also produced in neutrophils, albeit in low levels.22 Endogenously produced NO in neutrophils is likely to play an important role in signal transduction; several studies have documented the capacity of exogenously added NO to exert a variety of effects including inhibition of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, actin polymerization, and chemotaxis (see later). Excessive NO production has been implicated in many rheumatic diseases.23
Kinases and Kinase Cascades
There has been explosive growth in understanding of how kinases—proteins capable of enzymatically adding phosphate groups to target molecules—contribute to signaling in myeloid and nonmyeloid cells. Protein kinase C (PKC), actually a family of kinases, was among the first kinases implicated in neutrophil activation and is activated in response to chemoattractants. The ability of phorbol myristate acetate (PMA), a synthetic activator of PKC, to stimulate neutrophil responses including adhesion and O2− generation supports a role for PKC in neutrophil activation.24 In addition, inhibitors of PKC (including chelerythrine chloride and staurosporine) block stimulation of neutrophil functions.
The mitogen-activated protein kinases (MAP kinases) are a family of serine threonine kinases including the extracellular regulating kinase (ERK), p38, and c-Jun amino terminal kinase (JNK) families. In neutrophils, chemoattractants and other stimuli are capable of activating p38, JNK, and ERK, on time courses consistent with neutrophil activation. A role for ERK activation in signaling for both neutrophil O2− and in neutrophil adhesion and phagocytosis has been demonstrated.17,25 Phosphatidylinositol 3-kinase (PI3K) is a set of related enzymes that are found in abundance in neutrophils and primarily catalyze the phosphorylation, not of proteins, but of the 3-position of phosphatidylinositol phospholipids. One of the main bioactive products of PI3K is phosphatidylinositol triphosphate (PIP3). Chemoattractants such as N-formyl-methionyl-leucyl-phenylalanine (FMLP) rapidly activate PI3K in neutrophils, where it seems to play a role in diverse neutrophil functions including O2− generation, adhesion, and degranulation.26 PI3K also may regulate neutrophil survival and apoptosis.
Neutrophil Adhesion
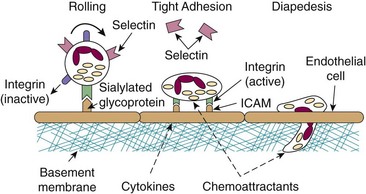
One of the earliest, crucial aspects of the inflammatory response is the ability of bloodstream neutrophils to adhere to vascular endothelium preparatory to movement into the tissues (Figure 11-4). Stimulated neutrophils also possess the ability to adhere to each other, a process termed homotypic aggregation, which may bring in vivo bloodstream neutrophils into proximity with neutrophils already adherent to the vessel or concentrate them at a site of inflammation. Extensive investigation has provided significant insight into the mechanisms involved in neutrophil adhesion. Several families of interacting adhesion molecules have been shown to exist on neutrophils and endothelial cells including the selectins, integrins, intercellular adhesion molecules (ICAMs), and sialylated glycoproteins.
The integrins are a large family of heterodimeric molecules generated by various combinations of α and β chains. Similar to the selectins, they require divalent cations (Ca2+ or Mg2+ or both) to engage their ligands. Neutrophils express three β2-type integrins, each constructed from a distinct α subcomponent (CD11a, CD11b, or CD11c) and a common β2 chain (CD18). Integrins use the ICAMs as their counterligands. CD11b/CD18 (also called Mac-1 or CR3) binds to fibrinogen, factor X, heparin, and the complement component iC3b in addition to ICAM and is most strongly implicated in neutrophil/endothelial and neutrophil/neutrophil interactions. In contrast to the selectins, neutrophil CD11b/CD18 is constitutively expressed but inactive; stimulation of neutrophils by chemoattractants and other agents results in changes in the activation state of CD11b/CD18 and increases its affinity for ICAMs and other ligands.27 Stimulation of endothelium with cytokines such as IL-1 results in increased expression of ICAM-1 and ICAM-2, providing a coordinate mechanism for the regulation of adhesion. In contrast to selectin-mediated adhesion, integrin/ICAM interactions are high-affinity and persistent. Stimulation of rolling neutrophils results in their tight adhesion to vessel walls and constitutes the first committed step in the movement of neutrophils into the tissues. Additionally, engagement of integrins by their counterligands sends signals into the cell (“outside in” signaling), regulating selective cell responses such as cytoskeletal reorganization, oxidant production, and degranulation. Outside-in signaling through CD11b/CD18 also coordinates with signaling through Fc receptor FcγRIII (see later) to mediate phagocytosis of particles opsonized by IgG and the complement component iC3b. Crosstalk between neutrophils and endothelial cells has also been shown to be a CD11b/CD18-dependent event: cross-linking of CD18 on neutrophils leads to increased permeability of endothelial cells, probably through the release of neutrophil proteases.28
The function of CD11a/CD18 on neutrophils has been controversial, but accumulating data indicate that this molecule may be necessary for neutrophil adhesion and emigration.29 The function of CD11c/CD18 is less clear, although it may play a role in neutrophil phagocytic activity.
Diapedesis and Chemotaxis
The mechanism by which neutrophils pass through the endothelial barrier is unclear. One report suggests a model in which neutrophils pass directly through pores generated within the endothelial cells themselves, but neutrophils alternatively may pass between endothelial cells by disruption of cell-cell junctions (Figure 11-5).30 Diapedesis occurs via homotypic interactions between adhesion molecules found on neutrophils and endothelial cells known as platelet-endothelial cell adhesion molecules (PECAMs). These molecules are concentrated at endothelial cell junctions, and antibodies that block PECAM inhibit transmigration in vitro by limiting neutrophils to the apical surface of the endothelium. Transmigrating neutrophils undergo upregulation of α6β1, an integrin that mediates binding to laminin (a key component of the perivascular basement membrane). Antibodies to α6β1 generally block neutrophil transmigration but fail to do so in a PECAM knockout mouse, implicating α6β1/PECAM as crucial to the passage of neutrophils out of the vasculature.31 CD47, otherwise known as integrin-associated protein, and CD99, expressed on neutrophils and endothelial junctions, have also been implicated in neutrophil passage through the endothelium.
When beyond the endothelium, most neutrophils pause for a time before traversing the basement membrane (basal lamina). Classic studies by Huber and Weiss32 suggest that neutrophils pass through the basement membrane via active disruption of its patency, without elucidation of known proteases or oxygen radicals. The disruptions are rapidly repaired by an unknown mechanism, probably involving the endothelium.
Chemotaxis in the direction of a gradient is achieved by the extension of membrane ruffles (lamellipodia), followed by anchorage of the ruffles to the substrate and withdrawal of the trailing edge of the cell in the direction of movement. These changes are accomplished primarily through rearrangement of the actin cytoskeleton after engaging chemotactic factor gradient that signals through GPCRs and PI3K. Actin is a 41-kD protein that exists as a soluble, globular monomeric form (G-actin) and as an insoluble linear polymer (F-actin). F-actin may be assembled (extended) at one end (barbed end) and disassembled at the other, under the control of regulatory molecules. During chemotaxis, F-actin formation and extension is concentrated at the leading edge of the neutrophil, permitting extension of the cell membrane (see Figure 11-5). Chemoattractant receptors also concentrate at the leading edge, defining the cell’s directional response to the gradient (headlight phenomenon). As the neutrophil moves along, receptors that were formerly at the leading edge are swept to the tail and internalized.
Phagocytosis and Degranulation
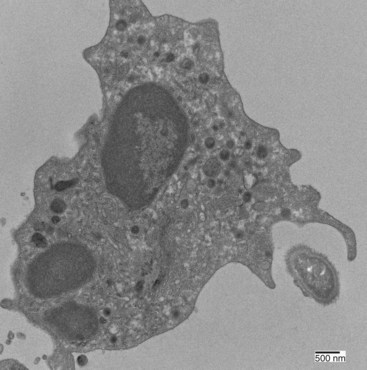
Neutrophil phagocytosis of an encountered bacterium or other particle requires direct contact. Neutrophils are generally poor at phagocytosing unmodified targets, particularly encapsulated bacteria (Figure 11-6). Two mechanisms for triggering phagocytosis have been defined. First, phagocytosis can be activated by direct neutrophil recognition of pathogen-associated molecular patterns, or PAMPs, which are small molecule motifs found on or in bacteria or viruses (but not typically mammalian cells). PAMPs are recognized by Toll-like receptors (TLRs)33 and other pattern recognition receptors (PRRs). Human neutrophils express all TLRs except for TLR3, and TLR activation stimulates human neutrophil phagocytosis by promoting structural and conformational changes.34 Second, phagocytosis also depends on opsonization (from the Greek, “to prepare for the table”), the modification of a target via its decoration with immunoglobulin or complement components or both. Neutrophils express two families of receptors for the Fc portion of complexed or aggregated IgG: low-affinity FcγRIIa and high-affinity FcγRIIIb. During some infections, or after in vitro stimulation with interferon or G-CSF, neutrophils also express the high-affinity receptor FcγRI, which binds monomeric IgG.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree