Vessel
Vulnerable areas
Injury
Mechanism
Subclavian vein
Medial third of clavicle
Perforation
Drill tearing
TOS
Callus compression
DVT
Subclavian artery
Junction medial and middle third
Pseudoaneurysm
Prominent metalware
Axillary artery and vein
Middle third clavicle
Pseudoaneurysm
Prominent metalware
AV fistula
Compression
TOS
Suprascapular artery
Superomedial glenoid
Perforation
Arthroscopic dissection
Cephalic vein
Anterior open or arthroscopic
Perforation
Instrumentation
Pseudoaneurysm
35.2.1 Mechanism of Injury
Blood vessel compromise may be the result of compression, traction, or perforation of the vessel wall. Perforation may involve a sharp (incision) or blunt force (laceration). The zone of injury may be narrow, such as a stab wound from a blade or wire, or wide, such as a tearing of the vessel over a sharp bone edge. The mechanism is usually acute, although some lesions, such as pseudoaneurysms , have been reported to be the result of attrition injury to the vessel wall over a prolonged period [8].
35.2.2 Pathoanatomy
35.2.2.1 Compression/Thoracic Outlet Syndrome
The thoracic outlet contains the neurovascular structures to the upper limb and can be divided onto three separate anatomical regions. Medially, the scalene triangle is the space occupied by the three scalene muscles (Scalenus anterior, medius and posterior). This space contains the brachial plexus and subclavian artery, which lie in the interval between the anterior and middle scalenes. The subclavian vein is not contained in this space because it lies anterior to the scalene muscles (Fig. 35.1). The costoclavicular space is the segment bounded by the clavicle superiorly, first rib inferiorly, scalenus muscles medially and pectoralis minor laterally. The retropectoralis minor (subcoracoid) space is lateral [9, 10].
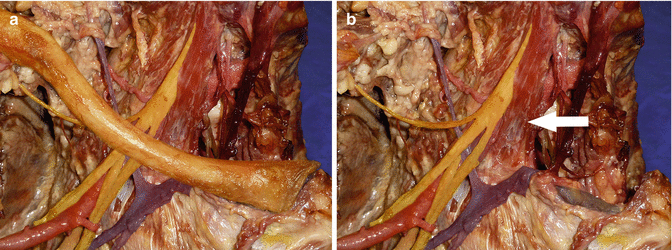

Fig. 35.1
Right anterior neck dissection with clavicle present (a) and clavicle removed (b). (a) Neurovascular structures adjacent to the medial two-thirds of the clavicle. (b) Brachial plexus and subclavian artery posterior to scalenus anterior (white arrow). Subclavian vein crossing first rib anterior to scalenus anterior and directly posterior to the medial clavicle and sternoclavicular joint (excised). At the medial end of the clavicle, the subclavian artery and brachial plexus are relatively protected by the scalenus anterior. White arrow–scalenus anterior muscle, colouring of the neurovascular structures has been enhanced to clearly demonstrate them. Subclavian/axillary artery: red Subclavian/axillary vein: blue Brachial plexus: yellow Sternoclavicular joint (excised): black (Copyright Dr Gregory Bain)
Thoracic outlet syndrome (TOS) may affect the vascular, as well as neurological, structures to the upper limb. Iatrogenic vascular TOS has been reported following plate fixation of clavicle fractures [11–13].
Iatrogenic TOS most commonly occurs in the costoclavicular space , as distinct from TOS of other aetiologies, which usually occurs more medially in the scalene triangle [9]. Compression of the vascular structures produces variable symptoms of discolouration, swelling and temperature changes, whilst brachial plexus compression produces variable pain, weakness and sensory disturbance. Significant callus formation may encroach upon the costoclavicular space (Fig. 35.2). In this situation, mobilisation and fixation of fragments may further reduce the volume of the costoclavicular space , to the point of a clinically apparent TOS [11, 12, 14].
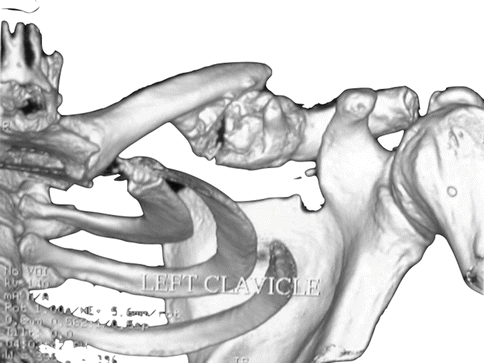

Fig. 35.2
Three-dimensional computed tomography reconstruction of left shoulder. Hypertrophic callus around a clavicular fracture site is demonstrated. This mass is decreasing the volume of the costoclavicular space (Copyright Dr Gregory Bain)
35.2.2.2 Perforation
The subclavian vessels are in close proximity to the medial two-thirds of the clavicle (Fig. 35.3). Injury to the subclavian vein has been reported following fixation of an acute clavicle fracture [15] and revision fixation for clavicular non-union [16]. The vein is thin walled, making it easily distendable but difficult to repair (Fig. 35.4). It is located a mean 5 mm from the posterior aspect of the medial clavicle, but can be directly adherent to the periosteum [17]. In contrast, the subclavian artery has comparatively thick walls and is relatively protected by the overlying scalenus anterior muscle.
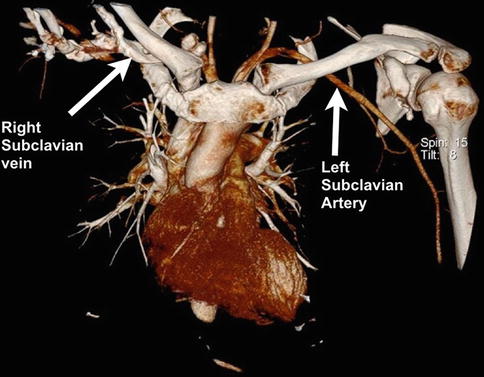
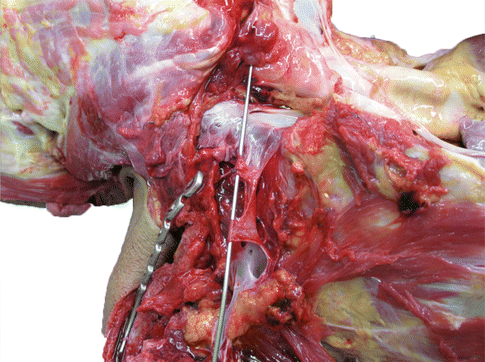

Fig. 35.3
Three-dimensional computed tomography angiogram of the mediastinum and thoracic outlet. Two of the subclavian vessels have been highlighted (right vein and left artery). Note the close proximity of the vessels to the medial end of the clavicle (Copyright Dr Gregory Bain)

Fig. 35.4
Post-mortem photo of lacerated subclavian vein which occurred during clavicle fixation. A probe is positioned in the lumen of the remaining vein. Note is made of the thin wall of the vessel and its proximity to the medial clavicle. The injury occurred during fixation of a clavicle fracture. Profuse bleeding was noted after one of the holes was drilled. The cause of death was blood loss and air embolism. The injury occurred despite the use of a periosteal elevator positioned along the inferior aspect of the bone, in an attempt to avoid plunging of the drill bit (Image courtesy of the Queensland coroner’s court, Australia, Used with permission)
35.2.2.3 Pseudoaneurysm
A pseudoaneurysm is an extravascular haematoma that freely communicates with the intravascular space through a defect in the vessel wall [18] (Fig. 35.5). The wall of a pseudoaneurysm is made up of compressed tissues surrounding the haematoma, rather than the three distinct layers of a true vessel wall [19, 20].
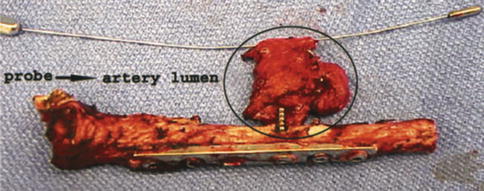

Fig. 35.5
Excised clavicle with pseudoaneurysm at the tip of the prominent screw. A dilator within the true lumen of the excised segment of the subclavian artery. Note risk factors; offending screw is markedly longer than the adjacent screw, screw protrudes from the postero-inferior surface of the middle third of the clavicle. Screw passage is eccentric, such that the surgeon may only have encountered one cortex when drilling (Used with permission from Shackford [22])
They are a result of penetrating trauma, with the most common iatrogenic cause being endovascular cannulation procedures. However, pseudoaneurysms have also been reported in the shoulder in association with prominent clavicular metalware [8, 21–23] (Fig. 35.6) and the following coracoid transfer procedures [2].
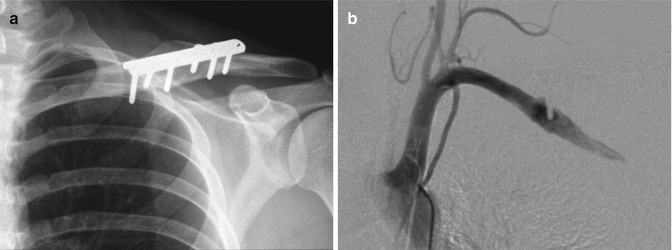

Fig. 35.6
(a) Plain radiograph of clavicle and fixation plate 6 years post-surgery for fracture. The patient had intermittent claudication symptoms in the ipsilateral upper limb for the preceding 18 months. (b) Angiogram of the same patient. A pseudoaneurysm is demonstrated around the tip of the most medial screw of the fixation plate (Copyright Dr Gregory Bain)
Pseudoaneurysms may be clinically silent for many years – a case associated with a prominent screw was reported as presenting 10 years following clavicle fixation [22] (Fig. 35.5). Intermittent, subacute ischaemic symptoms occur distal to the artery due to compression of the surrounding vessels. Thrombotic events can also occur, which may precipitate acute limb-threatening ischaemia.
35.2.2.4 Arteriovenous Fistula
Arteriovenous (AV) fistulae are a result of injury to both the artery and an adjacent vein [24]. Like pseudoaneurysms they are commonly associated with penetrating injury.
With time AV fistulae dilate and the pressure in the venous side of the lesion increases, causing venous engorgement and swelling. Persistent, untreated, venous hypertension can cause congestive heart failure or limb-threatening ischaemia.
35.2.2.5 Air Embolism
An air embolism occurs when air or gas is admitted into the vascular system [25] and it most commonly occurs with central venous catheterisation. It is also well recognised in posterior cranial fossa surgery [26], total hip arthroplasty and in spinal surgery in the prone position [27, 28]. Fatal air embolism has been reported following subclavian vein injury sustained during plate fixation of a clavicle fracture [15] (Fig. 35.4).
The surrounding soft tissues adherent to the vein can prevent it from collapsing, and the lumen of the vein has a negative pressure due to the negative intrathoracic pressure. Therefore, any breach of the vessel wall will potentially allow air to be sucked through the defect and into the vessel lumen [29].
Once air enters the subclavian vein , it can have several pathophysiologic consequences. The heart is designed to pump fluid, and struggles to pump the compressible air (“air lock”[30]), which can lead to hypoperfusion and even complete cardiovascular collapse [25, 27, 28, 31]. As in an embolism from any other cause, air in the pulmonary circulation leads to pulmonary vasoconstriction, release of inflammatory mediators, bronchoconstriction and ventilation/perfusion mismatch [27]. If there is a patent foramen ovale, the embolus has the potential to enter the cerebral circulation [27]. The lethal volume of air is estimated to be 200–300 ml (3–5 ml/kg). The closer the vein to the right heart, the smaller this volume [27].
35.2.2.6 Deep Vein Thrombosis
Deep vein thrombosis (DVT) is caused by the classic Virchow triad of vessel wall injury, altered blood flow and hypercoagulability [32]. Surgery and immobility are well known to increase the risk of DVT . There is one reported case of DVT following clavicle fixation [32]. However, it is unclear whether the DVT was due to the initial injury, the surgery or an underlying Paget–Schroetter syndrome. This is a form of thoracic outlet syndrome characterised by venous thrombosis [21, 32]. The risk of pulmonary embolism following upper limb DVT (3–36 %) is similar to that in the lower limb. However, the majority are asymptomatic and it is rarely fatal [33].
35.2.3 Presentation and Prognosis
The clinical effects of a perforation in a vein are usually observed at the time of surgery. Significant bleeding may be obvious; however, if the bleeding is into the chest cavity the only sign may be unexplained hypotension. The effects of air embolism are likewise rapidly apparent. If the cause of hypotension is not identified and addressed, they may prove fatal.
The thoracic outlet syndrome that occurs as a result of vessel compression is usually apparent in the immediate post-operative period.
Arterial wall injuries do not typically present at the time of surgery, but rather after a delay of months to years. Despite this long latent period, once the patient develops symptoms there is potential for them to deteriorate into frank upper limb ischaemia.
35.2.4 Specific Vessels at Risk
35.2.4.1 Subclavian Artery and Vein
The subclavian vessels are at risk during procedures on the medial and middle thirds of the clavicle. The subclavian artery is posterior to, and thus relatively protected by, the scalenus anterior muscle. In contrast the subclavian vein is anterior to this muscle and directly posterior to the medial clavicle. It lies a mean 5 mm from the bone but may directly appose to the posterior periosteum [17]. The vein wall is thin and can be injured by sharp bone fragments, retractors or drills and screws (Fig. 35.4).
35.2.4.2 Axillary Artery and Vein
The anatomical boundary between the subclavian and axillary vessels is the lateral border of the first rib. The axillary vessels converge and lie postero–inferior to the middle third of the clavicle at mean distance of 13–17 mm [17]. Pseudoaneurysms and AV fistulae of these vessels have been reported due to prominent metalware in this region.
The axillary artery descends on the chest wall to the lower border of teres major, where it becomes the brachial artery. Both axillary vessels are at risk in anterior approaches to the shoulder, particularly revision procedures where the anatomical relationships are distorted by scar tissue.
Anterior dislocation of the glenohumeral joint may cause the humeral head to be directly apposed to the axillary artery (Fig. 35.7). This alone may result in either early or late presenting vascular injury [34]. If an anatomic closed reduction cannot be achieved, then a vascular examination is mandatory to ensure that the artery is not interposed between the articular surfaces. Open reduction may be required in chronic cases, and special care must be taken to identify and protect the artery as it will be displaced into the surgical field.
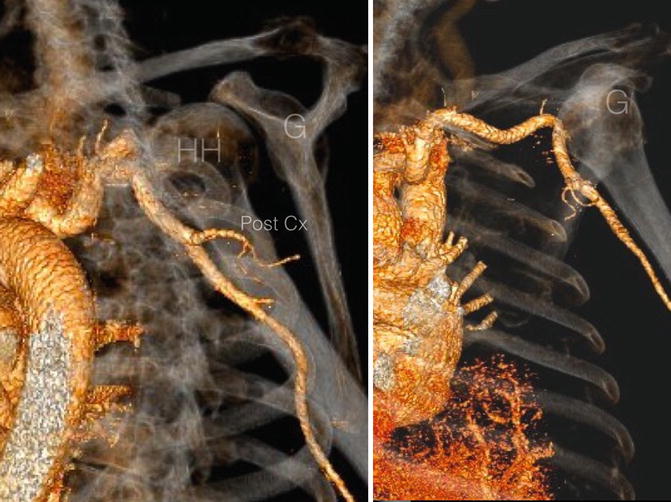

Fig. 35.7
Three-dimensional computed tomography angiogram of a 6-month old chronic anterior glenohumeral dislocation. The axillary artery is directly apposed to the anterior aspect of the humeral head and has been displaced anteriorly as a result. HH Humeral head, G Glenoid (Copyright Dr Gregory Bain)
Clavicular surgery warrants particular attention due to the close proximity of these major vessels to the operative field. Care must be taken when drilling to ensure that, wherever possible, the placement of drill holes should be along a trajectory that avoids the vessels. In the medial third of the clavicle, this safe trajectory is in a superior to inferior direction; and in the middle third, it is anterior to posterior [17]. Dissection around the medial two-thirds of the clavicle must be in the sub-periosteal plane to prevent injury to an adherent subclavian vein . The potential morbidity from compromised blood supply to the clavicle due to this dissection is far less than the morbidity of compromising blood flow to the upper limb or the right atrium due to a vascular injury .
35.3 Neurological Injury
35.3.1 Mechanism of Injury
Nerves may be injured by traction, compression or division. Division may be the result of blunt force (laceration), traction (avulsion) or contact with a sharp surface (incision). The extent of injury depends on the magnitude and duration of the provoking stimulus.
Traction injury occurs when attempts are made to mobilise, distract or retract a nerve, or structures surrounding a nerve, without adequately releasing adjacent tether points. These tethers may be the result of normal anatomy, such as where a nerve passes between two muscle heads, or pathological processes, where the nerve is adherent by scar to adjacent structures. Increasing the resting length of a nerve by 8 % has been reported to cause venous obstruction, with ischaemia occurring at 15 % [35].
Compression injuries may arise from inadvertent placement of retractors, interposition between a fixation plate and the bone or suture entrapment. Compression injury can also occur to prominent nerves that are outside of the shoulder region due to positioning of the patient without adequate padding.
35.3.2 Pathoanatomy
Two commonly used classification systems for nerve injury are based on the pathoanatomical lesion present. Seddon [36] classified nerve injury into three groups, depending on the integrity of the axon and the nerve. Sunderland [37] further delineated these groups according to the integrity of the various anatomical layers of the nerve (Table 35.2).
Sunderland classification (Seddon criteria) | Pathoanatomy | Prognosis |
|---|---|---|
I (Neuropraxia) | Focal conduction block | Recover in hours – weeks |
Axon intact | ||
II (Axonotmesis) | Axon ruptured | Recovery in months – years |
Endo, peri and epineurium all intact | ||
III (Axonotmesis) | Endoneurium ruptured, other layers intact | Recovery variable |
IV (Axonotmesis) | Perineurium ruptured but epineurium intact | Recovery variable |
V (Neurotmesis) | All layers ruptured – nerve divided | No recovery unless repaired |
The common feature of all cases of neurological injury is nerve dysfunction. If all axons are involved the lesion is complete, and if any axons are spared the lesion is partial.
35.3.3 Presentation and Prognosis
Pain is a feature of acute partial nerve injury. In cases where the patient has a persisting noxious stimulus to the nerve – such as an encircling suture or sustained traction over a short segment – the patient may wake from surgery with intense pain in the affected region [38, 39].
The prognosis of the dysfunction is related to the pathoanatomical lesion present in each nerve fibre, that is, the Sunderland class. This in turn directs the management of the injury. The pathoanatomy is influenced by the intensity and the duration of the injury mechanism.
Neurological lesions are usually transient and self-limiting, although permanent injuries have been reported. The brachial plexus or the axillary nerve are the most commonly affected structures. Recovery from a “transient” brachial plexus injury may take 6 months or longer, presenting a significant burden to the patient. Furthermore, it should be noted that the greater the degree of injury to the nerve, the less potential there is for recovery without surgical intervention.
35.3.4 Specific Nerves at Risk
A summary of the major nerves at risk, the areas where they are vulnerable and the reported mechanisms of injury is given in Table 35.3.
Table 35.3
Iatrogenic major nerve injuries about the shoulder
Nerve | Vulnerable areas | Injury mechanism |
|---|---|---|
Brachial plexus | Lateral position arthroscopy Middle third clavicle (postero-inferior surface) Anterior glenoid | In line traction at 45° abduction Iatrogenic thoracic outlet syndrome Mobilisation of fragments adherent to plexus Prominent metalware |
Axillary | Lower border subscapularis Inferior capsule Quadrangular space Proximal humerus | Antero-inferior portals, subscapularis dissection Capsular plication Capsular plication, posterior portals Compression under plate |
Suprascapular | Lateral clavicle Suprascapular notch (2.3 cm from articular surface) Base of scapular spine (1.4 cm from articular surface) Postero-inferior glenoid | Distal clavicle excision Arthroscopic dissection, glenoid instrumentation Posterior glenoid exposure Prominent metalware |
Musculocutaneous | Between heads of coracobrachialis | Coracoid transfer, biceps tenodesis, reverse TSA |
Spinal accessory | Sternocleidomastoid, posterior triangle of neck Superomedial angle of scapula | Lymph node surgery Arthroscopic portal placement |
Dorsal scapular | Superomedial angle and medial border of scapula | Scapulothoracic bursoscopy, medial scapular incisions |
Upper + lower subscapular | Anterior glenoid (18 mm medial to glenoid rim) | Subscapularis mobilisation during TSA |
35.3.4.1 Brachial Plexus
The brachial plexus is at risk from traction injury during arthroscopic procedures performed in the lateral decubitus position, with a reported 10–30 % incidence of transient paresthesias [40]. Traction is safest when applied with the upper limb positioned at either 0 or 90° of abduction, as the brachial plexus is under the least amount of strain in these positions [41].
Traction plexopathy is an important complication of reverse total shoulder arthroplasty (RTSA). The design of the prosthesis shifts the joint centre inferiorly, thereby increasing the distance between the acromion and the elbow. This has been reported to increase the strain on the plexus by 15–19 % [42].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








