111 Mycobacterial Infections of Bones and Joints
Another major force driving the increase in mycobacterial infection is the human immunodeficiency virus (HIV) epidemic that continues to be a worldwide problem. By 1991, 21% of extrapulmonary TB cases in the United States were associated with acquired immunodeficiency syndrome (AIDS). In developing countries, the HIV pandemic has led to marked increases in osteoarticular TB co-infection.1 These cases are distinguished by disseminated multifocal disease suggestive of hematogenous spread, rapid progression, and more common coexistence with pulmonary infection.2,3 HIV testing should be a routine part of the workup of any patient presenting with a musculoskeletal infection secondary to Mycobacterium tuberculosis. Since the introduction of effective antiretroviral therapy, the incidence of TB and nontuberculous mycobacterial infection in patients co-infected with HIV has sharply declined in the United States.
Although TB is uncommon among the non–HIV-infected population in the developed world (in the United States, TB is steadily declining among the general population), it remains a major problem in developing countries. There, TB continues to ravage the population, with 9 million new cases of active disease and 1.6 million deaths each year.4 Worldwide, it is the number two infectious disease killer after HIV/AIDS. Someone in the world is newly infected with TB bacilli every second. About one-third of the world’s population is infected with TB, providing a reservoir that will continue to complicate its global control.5 More alarming is the emergence of extensively drug-resistant TB (XDR-TB).6 These strains fail to respond to all first-line and most second-line TB agents. An outbreak of XDR-TB in South Africa was associated with a mortality rate of 100%, with a median survival of only 16 days after diagnosis. Globalization of the world economy is encouraging increased contact between populations of the developed and developing worlds. Recent immigrants to the United States from endemic areas constitute an expanding reservoir of patients with latent TB.
Clinical Scenarios
To appreciate the entire spectrum of clinical problems that a rheumatologist may encounter in dealing with mycobacterial infection, it is useful to think in terms of several distinct categories. Franco-Paredes7 and colleagues provided a clinically useful division of mycobacterial infections into four major categories. The following sections reflect that division.
Direct Involvement of the Musculoskeletal System
Constitutional symptoms are typically subtle or absent, and laboratory indicators of inflammation are often normal. Synovial effusion is frequently minimal, and the fluid, if it is attainable, shows nonspecific inflammation. Radiographic abnormalities may be delayed, although newer imaging techniques have allowed the earlier detection of abnormalities and the distinction of TB from other infections and neoplasm.8,9 Characteristic pulmonary or extrapulmonary findings are not always present; for example, less than 50% of osteoarticular TB presents with evidence of active or past pulmonary disease. Tuberculin skin tests (TSTs) and interferon-γ release assay (IGRA) testing may provide useful clues to the cause, but results are not invariably positive, especially in debilitated or immunosuppressed patients. Correct diagnosis is highly dependent, in most cases, on demonstration of the infectious agent by microscopic examination and culture of affected tissue.
In HIV-infected patients, mycobacterial infections are often diagnosed before patients’ HIV-positive status is known, sometimes leading to its recognition.10 Atypical pulmonary TB and extrapulmonary (often multifocal) infection are common; extrapulmonary infection occurs in 60% to 70% of such cases, compared with 16% of all TB patients.
The clinical patterns of musculoskeletal TB include spondylitis, osteomyelitis, peripheral joint infection, and soft tissue abscess. In a series of 230 consecutive cases of TB from the preantibiotic era, 5.2% had skeletal involvement; the spine was affected in 60% of these cases.11 TB of the bones and joints is spread hematogenously. The sites most commonly affected are the spine and hips, followed by the knees and wrists; other joint involvement is rare. Constitutional symptoms are unusual in musculoskeletal TB and, when present, suggest TB in other organs. Vertebral collapse due to spinal TB may initially be attributed to the more common osteoporosis-caused spinal compression fracture. TB only rarely involves skeletal muscle but must be considered in the differential diagnosis of an enlarging muscle lesion.12–16 Isolated cases involving tendons, trochanteric bursae, and fasciae latae illustrate the variety of possibilities.17–19 Biopsy and culture are required for diagnosis. Imaging studies do not reliably distinguish TB from neoplasm.
In nonendemic areas, skeletal TB usually occurs in elderly, debilitated patients, most often in the form of solitary osteolytic lesions in the axial skeleton. The development of skeletal disease is often remote from the initial infection, which strongly implies reactivation of previous subclinical disease. Patients may have false-negative TSTs for several reasons, including long-term corticosteroid use or coexisting debilitating disease, such as rheumatoid arthritis or chronic renal failure, which compromises resistance and produces anergy (Table 111-1).20,21 Spinal disease in children in nonendemic regions has largely been eliminated by effective medical therapy of pulmonary infection.
Table 111-1 Causes of False-Negative Purified Protein Derivative Test
In contrast, in endemic areas with high infectivity rates, those infected are more commonly children and young to middle-aged adults. These individuals have a higher incidence of multifocal skeletal involvement in the ribs, pelvis, vertebral appendages, cervical spine, feet, and long bone diaphyses, and they show positive TST reactivity.22 Bone seeding occurs through hematogenous spread, sometimes secondarily from another extrapulmonary site. When pulmonary findings are present, a miliary pattern is typical. Spread to bone may also occur from infected nodes, by direct extension or through draining lymph channels.23
Spondylitis
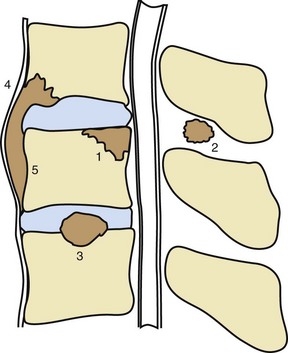
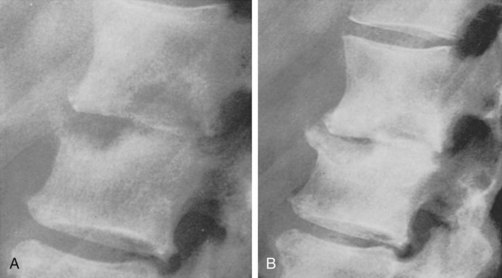
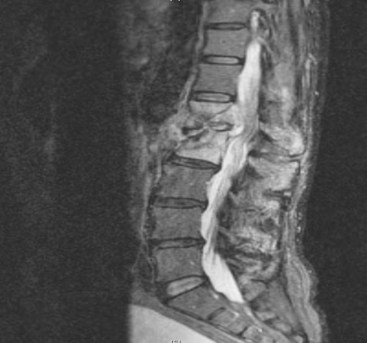
The spine is the dominant site of involvement in skeletal TB, accounting for 50% to 60% of cases.24 Between 48% and 67% of lesions occur in the lower thoracic and thoracolumbar spine in HIV-negative patients, whereas the lumbar spine is most commonly involved in HIV-positive patients.3 Infection usually begins in the anterior subchondral bone of a single vertebra adjacent to the intervertebral disk (Figures 111-1 and 111-2). Progression to bone takes 2 to 5 months and begins with extension from cancellous to cortical bone, and then across the disk space to adjacent vertebrae (Figure 111-3). Bone destruction may lead to vertebral collapse, which typically occurs anteriorly, resulting in gibbus deformity. Isolated neural arch involvement and intraspinal abscess may also occur.
A particular variant of this presentation is subligamentous TB, in which infection spreads up and down the spine beneath the longitudinal ligament, producing scalloping of multiple anterior vertebral bodies without disk involvement. This pattern is more common in the cervical spine.25
The clinical presentation of spinal TB usually consists of localized pain, which may be accompanied by low-grade fever, weight loss, chills, and nonspecific constitutional symptoms. Paraparesis and paraplegia have been reported in 1% to 27% of patients in various series. In comparison with pyogenic vertebral osteomyelitis, spinal TB more often presents with a prolonged clinical course, thoracic segment involvement, absence of fever, spinal deformity, neurologic deficit, and paravertebral or epidural masses.25 On occasion, tuberculous spondylitis may present with chronic inflammatory-type back pain more typical of a spondyloarthropathy.26
Mycobacterial colony counts in bone biopsy specimens are relatively low. Only 40% of smears and cultures from psoas abscesses are positive. Among patients meeting strict clinical and radiographic criteria in one series, between 73% and 82% had compatible histologic features on biopsy; of these, 80% to 95% had positive cultures.23 The differential diagnosis, which is extensive, includes pyogenic and fungal osteomyelitis, primary and metastatic tumors, sarcoidosis, multiple myeloma, and eosinophilic granuloma.
Cervical spine involvement is relatively rare, accounting for only 0.4% to 1.2% of cases of extrapulmonary TB in the United States.27 The most common presenting symptoms are neck pain and stiffness, although hoarseness, dysphagia, torticollis, fever, anorexia, and neurologic disorders may also occur. Spinal involvement can progress to myelopathy because of delays in diagnosis. Radiographs may show characteristic osteolysis of the anterior vertebral body with sparing of the posterior portion, gibbus deformity, disk involvement, and a partially calcified paraspinous mass. Computed tomography (CT) or magnetic resonance imaging (MRI) is useful for assessing compromise of the spinal canal. Retropharyngeal infection may extend into the craniocervical junction and, if not promptly recognized, may cause atlantoaxial dislocation and neurologic complications.28,29
The sacroiliac joint is involved in up to 10% of cases of skeletal TB, often without other evidence of disease.30 Infection, TB in particular, should be considered in all cases of unilateral sacroiliitis, particularly when other features of a spondyloarthropathy are absent. Emigration from an endemic area and a past history of TB increase the likelihood of this cause. Buttock pain on the involved side is the presenting symptom and is often accompanied by proximal leg or radicular pain. Examination reveals sacroiliac tenderness to palpation and stress maneuvers. Sacroiliac films show joint widening and erosion. An elevated erythrocyte sedimentation rate (ESR) and anemia are common, and a positive tuberculin reaction is typical. Biopsy of the sacroiliac joint shows granulomatous histologic features or nonspecific inflammation and a positive culture in most cases.
Atypical spinal lesions, which occur in about 10% of cases, may lead to delayed diagnosis and treatment. Atypical radiographic presentations in a single vertebra include concentric collapse, sclerotic foci, and selective involvement of the vertebral arches and costotransverse joints. Multiple vertebrae may be involved in continuity or as skipped lesions. Atypical clinical presentations may suggest a herniated intervertebral disk, failed back syndrome, spinal tumor, or a meningeal granuloma.24,31
Tuberculous Osteomyelitis
Bone lesions begin with hematogenous implantation of organisms in the medullary area. Metaphyseal involvement is most common, and lesions may spread through the growth plate to involve the adjacent joint, usually late in the disease course. Lesions are typically destructive. Lytic lesions in unusual areas, such as the pubic symphysis, sacroiliac joint, and elbow, can be misdiagnosed as malignancy.32 Osteomyelitis may develop in a bone or a joint that has been previously exposed to trauma.
Tuberculous osteomyelitis occurs in both children and adults.33 Although any bone can be involved, the femur and the tibia are most commonly affected. Dactylitis may also occur in children. In one large series from an endemic area, such cases represented 19% of bone and joint TB and 15% of cases of osteomyelitis of hematogenous origin.34 Bone pain was the most common presentation; a draining sinus, abscess formation, and local swelling and tenderness were also common. The average delay before diagnosis was 28 months.
Multifocal osteoarticular TB is a less common variant of the disease,34,35 but TB should be considered in all patients from endemic areas who present with multiple destructive skeletal lesions.
For a definitive diagnosis of osteoarticular TB, a biopsy specimen of an affected site must be obtained.18 Soft tissue lesions characteristically demonstrate rim enhancement on CT examination. CT may also facilitate percutaneous needle biopsy or abscess drainage.36 Histologic examination generally reveals granulomatous inflammation. In one series of 121 cases, biopsy showed a positive culture in 33%, granulomatous histologic features in 46%, and both in 21%.37 Radiographic findings include cavity formation with a thin adjacent layer of sclerosis in about 50% of cases, sometimes containing a sequestrum. The true extent of bone involvement may be difficult to detect because of clinically silent lesions. Bone imaging with technetium 99, although more sensitive than conventional radiographs, may provide false-negative information in early, indolent, or highly destructive disease. Tuberculin test reactions are positive in more than 80% of cases.38
Septic Arthritis
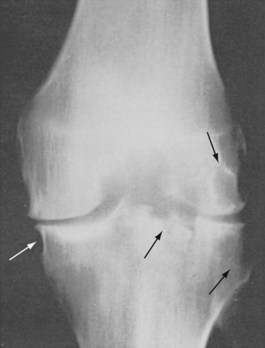
Tuberculous joint involvement is second in frequency to vertebral infection.24 The typical pattern is a monoarticular arthritis involving the large joints, most commonly the hip and knee (Figures 111-4 and 111-5).39,40 Other joints less commonly involved include the sacroiliac, shoulder, elbow, ankle, carpal, and tarsal joints. Infection begins in the synovium, with slower progression of destructive changes than in pyogenic septic arthritis. Prosthetic joint infection with M. tuberculosis has been reported and usually is caused by local reactivation of latent disease.41
The diagnosis of tuberculous arthritis is often missed. A consecutive series spanning the years 1970 to 1984 emphasized typical features.42 Of 23 cases of musculoskeletal TB, 9 involved the spine, 1 the hip, and the remaining 13 the peripheral joints. Most patients were men older than 50 years. The history of TB or exposure was generally forgotten. In all cases, presenting symptoms included joint pain and swelling. Four patients had evidence of active pulmonary TB, and in two patients with sterile pyuria, M. tuberculosis grew from the urine. Only 5 of 10 patients tested had a positive TST. Radiographs showed changes of erosive arthritis in 7 and no changes in 4 of 11 joints studied. The median delay in diagnosis was 8 months.
Arriving at a correct diagnosis requires vigorous pursuit and usually a synovial biopsy and culture. Initial studies are often misleading and may contribute to delayed diagnosis or misdiagnosis. Synovial fluid findings are variable and do not distinguish this arthropathy from other inflammatory or septic arthritides.43 Cell counts more often suggest bland inflammatory, rather than septic, arthritis and may contain a preponderance of neutrophils. The diagnosis may be facilitated if the organism is observed on an acid-fast smear of synovial fluid, but only 10% to 20% of reported cases are positive. In contrast, synovial fluid cultures are frequently positive. Radiographic changes are similar to those seen in other septic arthritides, beginning with juxta-articular bone demineralization and progressing to marginal bone erosion and articular cartilage destruction (see Figure 111-4).
With the open biopsy technique, granulomatous histologic features and positive cultures are present in more than 90% of cases.43 No data are available for direct amplification tests in mycobacterial arthritis, but their use can be considered in suspected cases to arrive at an earlier diagnosis. The histologic examination alone may be confusing, because granulomatous synovitis can also be found in nontuberculous mycobacterial infection, sarcoidosis, erythema nodosum, brucellosis, Crohn’s disease, and foreign body reaction. As noted, synovial acid-fast smear is of limited value. Tuberculous arthritis is also reported in children, sometimes early in the disease course; synovial biopsy and culture are recommended in children with monoarthritis and a positive tuberculin reaction.44
TB must be considered among the possible causes of septic arthritis in patients with pre-existing rheumatoid arthritis, although it is not widely seen in developed countries.45,46 Conversely, rheumatoid factor may be present in TB, leading to diagnostic confusion in the presence of chronic monoarthritis.45
Emergence of Tuberculosis during Treatment of Rheumatic Disease
Many patients with systemic rheumatic disease have dysregulated immune systems that are treated with immunosuppressive drugs. Such an impaired immune response may permit the reactivation of latent TB. TNF plays a key role in granuloma formation and stabilization, which promotes the containment of M. tuberculosis. The rate of TB among patients with rheumatoid arthritis before the widespread use of anti-TNF drugs was approximately 6 cases per 100,000,47 although some studies have suggested that the rate in rheumatoid arthritis is higher than in the general population.48 In one large patient registry, the estimated incidence of TB associated with infliximab in rheumatoid arthritis patients was in excess of 1000 per 100,000 person-years of exposure during the years 2000-2001.49 In the United States, the rate was 52 per 100,000 person-years during the same time period.47 Etanercept may be associated with a lower risk for TB reactivation than infliximab: 10 cases per 100,000 person-years of exposure with etanercept, versus 41 per 100,000 person-years with infliximab, according to an analysis of Food and Drug Administration data.50 More recently, both British and French registries of biologic use have identified a greater risk for TB associated with the use of adalimumab and infliximab than with etanercept.51,52 Fewer data are available for the newer TNF antagonists, and comparisons are complicated by the widespread use of pretreatment screening instituted after the first agents in this class were approved for use. Monoclonal antibodies to TNF may play a greater role in destabilizing granulomata than TNF receptors. Nonetheless, similar precautions should be taken with any TNF antagonist, and all should be presumed to increase risk of new infection and reactivation of latent infection.
Other biologic agents may pose a lower risk for reactivation of TB because the mechanisms of action are not intimately involved with host defenses related to intracellular organisms. Animal studies have suggested that abatacept does not impair the ability of mice to respond to M. tuberculosis, but this has not been confirmed in humans.53 Patients treated with abatacept and tocilizumab in clinical trials were prescreened with a TST and excluded if positive, thus making a direct comparison difficult. Little or no evidence indicates that B cells play a major role in containing TB, and no recommendation for TB screening is included in the labeling for rituximab.
Glucocorticoid use poses a significant hazard for patients with latent TB. Many mechanisms account for this effect, such as impairment of cellular immune responses and monocyte chemotaxis and function, including the monocyte’s production of TNF. The use of glucocorticoids has been associated with a five times increased risk for the development of TB in a case-control study based on a large general practice database in the United Kingdom.54 The risk appeared to be dose related but was seen even at the physiologic dose of prednisone 7.5 mg/day. Just as with anti-TNF therapy, the risk for TB was greatest early in the course of treatment. In a more recent study from France, prednisone given at 10 mg or more per day, but not at lower doses, was associated with an increased risk for developing TB.52
Reactivation of TB in the face of anti-TNF therapy typically occurs within 6 months of treatment and often presents as extrapulmonary disease. Institution of a TB screening protocol in rheumatoid arthritis patients and treatment of latent TB before TNF antagonist administration resulted in a 78% decrease in active TB.55 Strategies to treat latent TB infection that are tailored to the at-risk population can effectively and safely lessen the likelihood of active TB in patients treated with TNF antagonists. Indeed, it has been suggested that much of the blame for the development of active TB in this situation can be attributed to incomplete compliance with screening protocols.56 Conversely, in a report of 84 patients at a single center treated with etanercept after demonstrating a positive purified protein derivative (PPD), no cases of active TB were seen during a mean of 2 years of follow-up, despite the fact that only 78 patients received prophylaxis, and 26 of those failed to complete the prescribed course.57 Although screening for latent TB infection has reduced the incidence of active disease, false-negative TSTs can undermine such good intentions (see Table 111-1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree