10 Mononuclear Phagocytes in Rheumatic Diseases
Macrophages, dendritic cells, and osteoclasts are derived from blood monocytes.
Distinct subsets of monocytes are recruited to inflammatory sites in tissues.
Mononuclear phagocytes are widely distributed, biosynthetically active cells derived from hematopoietic precursors that circulate as monocytes and enter tissues constitutively and in response to inflammatory stimuli. In connective tissue and bone, they play a major role in homeostasis, growth, and remodeling as mature macrophages and osteoclasts (Figure 10-1). Myeloid dendritic cells (DCs) represent a distinct form of differentiation, specialized to maintain immune tolerance or to induce humoral and cellular immunity through B lymphocytes and T lymphocytes. Through their recognition, antigen-presenting, regulatory, and effector mechanisms, mononuclear phagocytes contribute to a range of inflammatory, infectious, autoimmune, metabolic, and degenerative rheumatic diseases, providing targets for therapeutic intervention.
Growth of knowledge in mononuclear phagocyte biology has developed in close association with understanding of pathogenesis and treatment of chronic arthritis. Relevant examples include the development of steroidal and nonsteroidal anti-inflammatory agents and of anti–tumor necrosis factor (TNF) monoclonal antibodies.1 Genetic lesions in macrophage colony-stimulating factor (M-CSF) cause osteoclast deficiency and osteopetrosis in mouse models, whereas human mutations in cytosolic nucleotide oligomerization domain (NOD)-like receptors (NLRs) result in interleukin (IL)-1β overproduction and hyperinflammatory syndromes,2 often associated with persistent joint disease. Immune complex deposition and complement activation combine with Toll-like receptor (TLR) recognition to induce effector pathways of tissue destruction and repair.
This chapter reviews general aspects of mononuclear phagocyte differentiation, recruitment, and activation, integrating the properties of different cellular subtypes. Studies on the biology of macrophages, DCs, and osteoclasts have diverged to the extent that common features have been overlooked in the understandable search for specificity. This review is an attempt, in part, to reintegrate these differentiated sublineages. General features especially relevant to physiologic and pathologic consequences of their presence in bone, joints, and connective tissues are discussed, and gaps in our knowledge are pointed out. The emphasis is on studies in humans, where available, with reference to murine models where applicable. The subject area encompasses innate and acquired immunity, autoimmunity, and osteoimmunology,3 a reflection of local specialization of mononuclear phagocyte and lymphocyte biology. Relevant topics discussed elsewhere in this textbook include innate immunity (see Chapter 18), cytokines and chemokines (see Chapter 26), osteoclast functions (see Chapter 4), and anti–TNF therapy (see Chapter 63). For further details in connection with macrophage4–7 and DC biology,8–10 see reviews cited in this chapter.
Overview
Circulating monocytes give rise to tissue macrophages, which display considerable phenotypic microheterogeneity in different organs.7 Similarly, and with some overlap in properties, myeloid DCs are present as heterogeneous sentinel cells at mucosal and cutaneous surfaces,9–11 undergoing a complex maturation process as they migrate to lymphoid organs after capture of antigens. Plasmacytoid DCs may represent a distinct sublineage, specialized to produce high levels of type I interferon in response to viral stimuli. Mononuclear precursors in the blood give rise to multinucleated osteoclasts, specialized to resorb bone.12 Circulating monocytes are themselves heterogeneous, using distinct chemokine and adhesion receptors to give rise to different tissue mononuclear phagocytes.13 These cells differ in life span depending on the recruitment stimuli and on local factors in their environment, and they express diverse plasma membrane receptors, making it possible for them to interact with many different cell types and with microbial and modified host components. Phagocytosis is a hallmark of the ability of macrophages and DCs to engulf particulates, including foreign materials, bacteria,14 and dying host cells generated by apoptosis or necrosis.15
These particulates are recognized by nonopsonic receptors, including scavenger and lectin-like receptors, or after opsonization with antibodies or complement or both that enhance uptake via Fc and complement receptors. In addition, other humoral proteins, such as Pentraxins, interact with their target ligands,16 bridging them to less well-defined macrophage receptors, which regulate early cellular responses during innate immunity. TLRs play an important role in sensing the nature of the captured cargo, often interacting with the extensive repertoire of non-TLR receptors (NTRs). An extended family of NOD-like receptors (NLRs) senses various cytosolic ligands, resulting in a complex assembly of proteins (inflammasomes), caspase activation, and release of IL-1β.17 Uptake of particulate and soluble antigen, directly or by “cross-priming,”18 induces DC maturation, processing, and association with major histocompatibility complex (MHC) II molecules, as well as presentation of peptides to naive CD4 T lymphocytes. Endogenously generated or foreign peptides generated during biosynthesis of virus glycoproteins associate with MHC I molecules for surface recognition by cytotoxic T cells (mainly CD8). DCs and possibly macrophages regulate T cell activation or tolerance through additional co-stimulatory surface antigens and cytokines, depending in part on concomitant TLR stimulation. Activated T lymphocytes and their products, such as interferon-γ, IL-4/IL-13, and IL-10, regulate the effector functions of mononuclear phagocytes.
The secretory activities of mononuclear phagocytes influence a range of cellular and extracellular targets to maintain tissue homeostasis but also are responsible for tissue destruction. Their trophic actions,19 through cellular contact with other stromal cells and extracellular matrix and secretion, regulate tissue catabolism, cell growth, angiogenesis, and repair. In addition to local effects, macrophages contribute to systemic integration of proinflammatory and anti-inflammatory effects, acting on the central nervous system, endocrine organs, liver, and energy stores.
Overall, the activation phenotype of mononuclear phagocytes is modulated by extrinsic factors (e.g., cytokines, hormones), by balance of surface receptors with activating/inhibitory cytoplasmic motifs, by cytosolic regulators such as suppressors of cytokine synthesis (SOCS)20 proteins, by phosphorylation/dephosphorylation of signaling molecules, and by assembly of transcription factor complexes on chromatin. Microarray analysis of macrophages has made it possible to distinguish characteristic signatures of gene expression after innate activation via TLRs, deactivation via glucocorticoids, or modulation via cytokines. As a result, the phenotype of tissue macrophages is markedly heterogeneous, making for complexity of function in different sites in health and disease, but also providing opportunities for novel state-specific or tissue-specific targeting by drugs.
Life History and Heterogeneity (Macrophages, Dendritic Cells, and Osteoclasts)
In an adult, all three sublineages of mononuclear phagocytes originate from CD34+ committed progenitor cells in the bone marrow (Figure 10-2). These diverge from lymphoid cells and subsequently from polymorphonuclear leukocytes, although many genes are still expressed but not translated in both types of phagocytic cell. In vitro, bone marrow and blood monocytes can be stimulated by growth factors21 to generate macrophages (M-CSF or granulocyte-macrophage colony-stimulating factor [GM-CSF]), DCs (GM-CSF, with or without IL-4), or osteoclasts (M-CSF and RANK ligand). Some of these growth factors also are essential in vivo (e.g., osteopetrotic, M-CSF–deficient mice lack many but not all populations of tissue macrophages and osteoclasts).22,23 Monocytopoiesis is less well understood in humans and may depend on M-CSF and GM-CSF. Trophic interactions between hematopoietic precursors and stromal cells in the bone marrow (mesenchymal and hematopoietic in origin) are mediated by cell contact, via surface receptors,19 and by soluble factors (e.g., c-kit ligand and IL-1). Transcription factors that are essential for monocyte/macrophage production and related cells include Pu-1, other Ets family members, Maf,24 and Mi, implicated in microphthalmia.
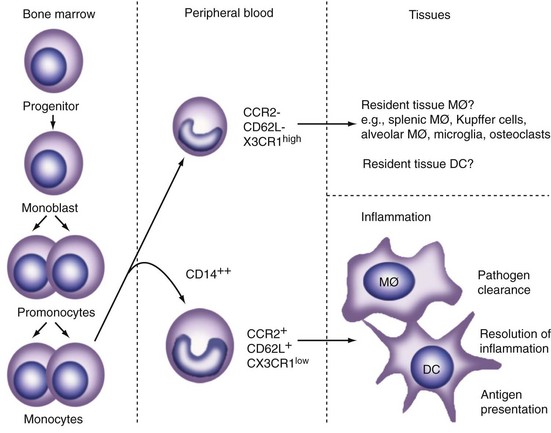
Figure 10-2 Differentiation and distribution of mononuclear phagocytes. Distinct subpopulations of circulating monocytes are thought to give rise to resident tissue macrophages (MØ), dendritic cells (DCs), and osteoclasts compared with cells recruited by an inflammatory stimulus. Further phenotypic heterogeneity arises from microenvironmental stimuli, such as cytokines and microbial products. For additional details of morphologic and other properties of cells in different tissues, see Gordon6 and Gordon and Hughes.7
Although bone marrow precursors proliferate vigorously as they differentiate in the presence of M-CSF or GM-CSF, monocytes become refractory to these growth stimuli. Local macrophage proliferation can be induced by IL-4 during Th2 inflammation.24a Restriction of DNA synthesis is associated with chromatin condensation, whereas RNA and protein synthesis persists and can be modulated by a variety of stimuli, as is discussed further subsequently.
Tissue macrophages, DCs, and osteoclasts all derive from circulating monocytes, although these already may display heterogeneity (see Figure 10-2). Recent studies in transgenic mouse models and in humans have described progenitor cells and precursor-product relationships in monocyte-macrophages, as well as DC differentiation.11 Cells are recruited constitutively to peripheral sites in the steady state. A subset of patrolling monocytes may not leave the vasculature in the steady state. Additional monocytes can be recruited to local sites in response to infectious, inflammatory, and metabolic stimuli. Such “elicited” cells display distinct properties from the “resident” cells, which, in the case of macrophages and DCs, also display marked diversity, depending on their location. A considerable body of evidence indicates that different subsets of macrophages and DCs originate from distinct monocyte populations in peripheral blood.25–28 Apart from marker antigens (e.g., CD14, the receptor for lipopolysaccharide-binding proteins; Gr-1, a mouse Ly-6 antigen expressed by polymorphonuclear neutrophils and by some monocytes), levels of chemokine receptors for fractalkine (CX3CR1) and CCR2 (MCP1) seem to distinguish monocyte subsets that give rise to inflammatory and resident macrophages (see Figure 10-2). Resident macrophages and immature DCs are present in many lymphohematopoietic and nonlymphoid organs; selected markers and properties are listed in Table 10-1. The CD68 antigen, a late endosomal mucin-like glycoprotein related to the lysosome-associated membrane protein (LAMP) family, is the most broadly expressed marker for all mononuclear phagocytes, although its function is still obscure.
Table 10-1 Selected Properties of Mononuclear Phagocytes and Related Cells
| Monocytes/Macrophages: Antigen Marker |
| Myeloid Dendritic Cells: Antigens |
| Plasmacytoid Dendritic Cells: Antigens |
| Osteoclasts |
Note: Marker expression varies, depending on cell localization, maturation, and activation. Some markers also are present on other myeloid cells (e.g., polymorphonuclear neutrophils) and on selected endothelial cells. Structures and functions of receptor antigens are described elsewhere in this chapter.
Apart from the expression of chemokine receptors,29 adhesion molecules play a role in selective monocyte “homing,” but their differential expression by macrophages, DCs, and osteoclasts is less well defined than for lymphocyte subpopulations. These include various heterodimeric integrins implicated in adhesion to endothelium, to extracellular matrix, and to bone. There is considerable scope for characterization of additional receptors and markers; one example is the EGF-TM7 family of leukocyte receptors illustrated in Figure 10-3.30 Although the F4/80 antigen has been extremely useful as a differentiation marker in the mouse, the human counterpart (EMR1) has not been of comparable value, showing a predilection for eosinophils. EMR2, a related human myeloid cell receptor, is a useful marker, however, expressed by many tissue mononuclear phagocytes; it binds chondroitin sulfate proteoglycans broadly present in connective tissue and has been implicated in leukocyte adhesion, migration, and activation.31
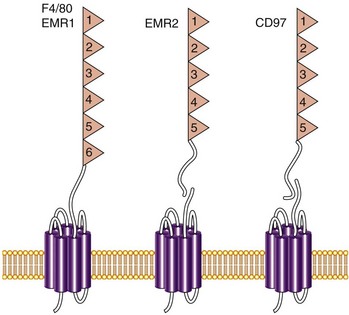
Figure 10-3 Myeloid cell antigens of the EGF-TM7 family. These G protein–coupled receptor (GPCR)-related receptors have a large extracellular domain consisting of multiple epidermal growth factor (EGF) modules. The F4/80 antigen, an excellent marker for mouse macrophages, has been implicated in peripheral tolerance.95 The human orthologue EMR1 is a marker for human eosinophils. EMR2 is not expressed in the mouse but is present on human monocytes, macrophages, immature myeloid dendritic cells, and activated polymorphonuclear neutrophils. It is a useful surface marker for macrophages in tissues, including rheumatoid arthritic joints. CD97, expressed on myeloid cells and selected nonmyeloid cells, is a receptor for the complement regulatory protein, CD55.96 EMR2 and CD97 bind chondroitin sulfate B.31
Mobilization of Mononuclear Phagocytes
Similar to other white blood cells, mononuclear phagocytes are distributed in intravascular and extravascular compartments, sharing common mechanisms of mobilization but also displaying distinct features among themselves and compared with other cell types. Exit from the bone marrow is controlled constitutively and on demand, but this process is not well understood, except for the role of chemokine receptors, such as CCR2 (ligands MCP1 through MCP4).32 What determines differentiation into the mature macrophages that form part of the stromal microenvironment in bone marrow is unknown; one possibility is re-entry of circulating monocytes from blood to bone, as for osteoclasts. Apart from the circulating pool of monocytes, many mature macrophages adopt a sinusoidal distribution in liver (Kupffer cells) and in selected lymphoid and endocrine organs. These cells are distinct from, but share endocytic properties with, sinusoidal endothelial cells. The constitutive exit through vascular endothelium to become tissue macrophages and DCs is not understood, whereas induced mobilization is well characterized, sharing many features with polymorphonuclear neutrophils.
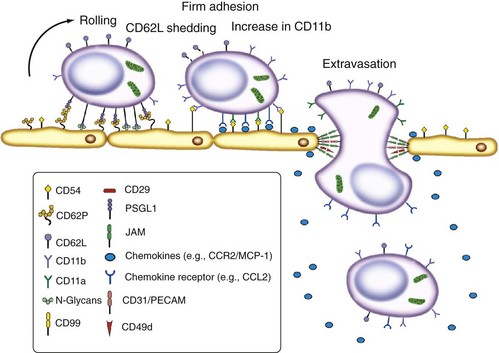
Figure 10-4 summarizes stages and molecules implicated in monocyte egress. Although most monocytes exit the microvasculature by diapedesis between endothelial cells, evidence of an alternative transcytotic mechanism is present, as for lymphocytes. The roles of l-selectin, β2, and other integrins, the immunoglobulin superfamily molecule CD31, and CD99 have been established by analysis of genetic deficiencies in humans and mice,33 and by the use of monoclonal antibodies against these34 and other defined adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) and very late activation antigen-4 (VLA-4). Endothelial cell ligands implicated in monocyte adhesion include fractalkine, a tethered chemokine; other chemokines may be presented by glycosaminoglycans.
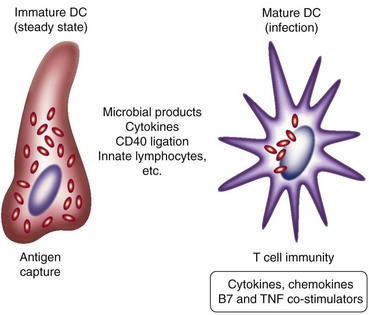
The subsequent migration and fate of mononuclear phagocytes in tissues differ strikingly. Although many resident macrophage and DC subpopulations in tissues are well characterized,7,35 the origins of several functionally specialized cell types related to this overall lineage remain unclear.36 Macrophages become sessile but can be induced by inflammatory stimuli to migrate to draining lymph nodes and remain there, without re-entering the circulation. Immature DCs respond to antigenic and inflammatory stimuli by migrating to draining lymphoid tissues, thereby transporting antigens for presentation to lymphocytes. The subcapsular sinus macrophages of lymph nodes have been implicated in trapping of DC antigen transported by afferent lymph37 (Figure 10-5). DC maturation is accompanied by major changes in DC properties (Figure 10-6 and Table 10-2). DCs become highly mobile and express a range of chemokine and adhesion receptors. Entry of myeloid DCs and tissue macrophages into lymphatics is less well understood but may involve interactions with the mannose receptor. As a result, DCs can present exogenous antigens and self-antigens to CD4 T lymphocytes to activate or tolerize their responses. Although in vitro systems are widely used to generate DCs by cultivation of monocytes or bone marrow precursors in cytokine-supplemented media (GM-CSF, with or without IL-4), their properties may not correspond to those of DC populations in vivo.
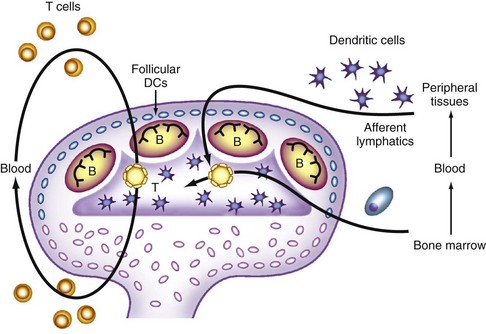
Figure 10-5 Positioning of dendritic cells (DCs) within lymphoid tissues. Blood-derived monocytic precursors enter skin and mucosal peripheral tissues and differentiate into Langerhans cells within epithelia and into other immature DCs. When established, Langerhans cells turn over independently from bone marrow–derived cells42 (e.g., after ultraviolet irradiation). On exposure to stimuli (e.g., foreign antigens, local infections) and constitutively (after uptake of apoptotic cells, such as in the gastrointestinal tract), DCs undergo maturation, upregulate CCR7, and enter afferent lymphatics and secondary lymphoid tissues, where they interact with CD4 T lymphocytes. CD4 T lymphocytes become activated, interacting with B cells or CD8 T lymphocytes, and re-enter blood, homing to peripheral sites. DCs also are able to interact with innate lymphocytes and natural killer cells. Follicular DCs in B cell areas have a distinct, poorly defined bone marrow origin, express novel antigenic markers, and are able to capture immune complexes through complement activation. Plasmacytoid DCs have a distinct interfollicular location and express markers of a myeloid and a lymphoid nature.
(Courtesy R. Steinman.)
Table 10-2 Maturation Markers for Human Monocyte–Derived Dendritic Cells
| Immature | Mature | |
|---|---|---|
| Enhancer/Co-stimulatory Molecules | ||
| CD80 (B7-1) | Low | High |
| CD86 (B7-2) | Low | High |
| CD83 | De novo | |
| EMR2 (CD312) | High | Moderate/low |
| Antigen Uptake | ||
| FcγRII | High | Low |
| Signaling | ||
| CD40 | Low | High |
| CXCR4 | Low | High |
| CCR5 | High | Low |
| CCR6 | High | Low |
| CCR7 | Low | High |
| Antigen Presentation | ||
| HLA-DR (major histocompatibility complex class II) | Moderate | High |
| HLA-DQ (major histocompatibility complex class II) | Moderate | High |
| CD1a | High | Low |
| Others | ||
| DC-SIGN (CD209) | High | Low |
| CD14 | High/moderate | Negative |
| CD123 (IL-3R) | Low | High |
Note: In addition to the listed antigens and antigen-presenting cell functions, dendritic cell production of, and response to, other growth factors, chemokines and cytokines, microbial products, and immune complexes varies during maturation and activation in vitro. Validation in vivo is incomplete.
Mononuclear phagocytes use integrins and receptors for extracellular matrix such as CD44, regulating the dynamics of their adhesion and migration. Osteoclast adhesion to bone depends on αvβ3 and possibly other adhesion molecules implicated in podosome attachment. Plasma membrane receptors such as CD97 and EMR2, members of the EGF-TM7 family, and TREM-1,38 an immunoglobulin superfamily molecule with an immunotyrosine activation motif (ITAM)-based activation motif (see later), regulate myeloid cell effector functions and adhesion and migration within extravascular tissue compartments.
Monocytes recruited to tissues by poorly degradable foreign materials or by selected microbial pathogens or parasites form granulomas, organized structures rich in macrophages, incorporating other myeloid and lymphoid cells and fibroblasts and extracellular matrix. Pathogen-induced granuloma formation39 depends on adhesion molecules such as CR3, a β2 integrin, TNF, and chemokine receptors such as CCR4; granuloma macrophages express abundant secretory products such as lysozyme40 and proinflammatory cytokines. Characteristic morphologic evidence of macrophage differentiation in granulomas includes epithelioid cell and multinucleated giant cell formation, such as with Mycobacterium tuberculosis infection. Giant cells arise by monocyte recruitment and macrophage fusion, rather than by impaired cytokinesis.41,41a Cytokines such as IL-4 and IL-13, acting through a common receptor chain, and GM-CSF promote macrophage fusion. Foreign surfaces including biomaterials also can play a role in granuloma formation, and chemokine receptors have been implicated in the induction of foreign body–induced giant cells.
The surface molecules involved in these examples of macrophage homokaryon formation and the functional significance of induced fusion are still poorly understood. By contrast, multinucleation in osteoclasts is a physiologic process, dependent on M-CSF and RANK ligand, and several plasma membrane (CD44, CD9, TREM-2, DC-STAMP) and intracellular (c-src, c-fos) molecules have been implicated in this differentiation process. Multinucleation is thought to favor efficient localized resorption of bone. Osteoclasts become polarized for secretion and display highly active plasma membrane ruffling. Interactions with osteoblasts; local cytokines, such as osteoprotegerin; and circulating hormones, including calcitonin, parathormone, and vitamin D metabolites regulate their gene expression and function, as is discussed in Chapter 4. Vitamin D receptors also modulate the function of macrophages and DCs.
The turnover of different mononuclear phagocytes varies, depending on their activation status and tissue localization.42 Resident macrophages can live for weeks or months, whereas inflammation reduces survival to hours or days. DCs are relatively short-lived cells; osteoclast turnover in vivo has not been studied in detail. The role of apoptosis in mononuclear phagocyte turnover is poorly characterized, in contrast to that in polymorphonuclear neutrophils, but cell survival is regulated by growth factors such as M-CSF and by interactions with neighboring cells and pathogens.