110 Lyme Disease
Lyme disease is a multisystem disorder caused by the tick-borne spirochete Borrelia burgdorferi.1 The disease first came to medical attention in the United States in the late 1970s with the investigation of a clustering of cases of juvenile arthritis in the region of Lyme, Connecticut.2 A characteristic skin rash described as single or multiple expanding red macules often heralded the onset of arthritis.2 This rash, termed erythema migrans (EM), had been linked in Europe to the bite of Ixodes ticks and the subsequent development of neurologic abnormalities.3,4 Further investigation revealed that arthritis was one manifestation of a systemic disorder affecting the skin, heart, joints, and nervous system. In 1982 Burgdorfer isolated the causative agent, the spirochete B. burgdorferi, from Ixodes ticks.5 Demonstration that patients with Lyme disease developed antibodies to this organism and its eventual culture from skin, cerebrospinal fluid (CSF), and synovial tissue confirmed the infectious etiology of the disorder.6 It is now the most common vector-borne disease in the United States.1
Ecology and Epidemiology of Lyme Disease
Lyme disease has a worldwide distribution, with most cases reported in North America, Europe, and Asia.7 On each of these continents, hard-shelled ticks of the Ixodes family are the only known vectors for the disease. Other arthropods and blood-sucking insects such as mosquitos cannot transmit the infection. The incidence of Lyme disease varies geographically and is determined by the prevalence of B. burgdorferi–infected ticks. In the United States, cases of Lyme disease have been reported in all 50 states and the District of Columbia, but most are clustered in the Northeast and mid-Atlantic region, upper Midwest, and northern California. In 2009, 29,959 confirmed and 8509 probable cases were reported to the Centers for Disease Control and Prevention, with 93% of confirmed cases originating from 11 states: Pennsylvania, New Jersey, New York, Massachusetts, Connecticut, Wisconsin, Maryland, Minnesota, New Hampshire, Delaware, and Maine.8
The spirochetes associated with Lyme disease reside within the genus B. burgdorferi sensu lato (sl), and the vast majority of cases are caused by B. burgdorferi sensu stricto (ss), Borrelia garinii, and Borrelia afzelii.7 All three genospecies can be found in Europe, whereas B. burgdorferi ss is the main species found in North America. Variation among the genospecies may account for the differences in clinical expression of Lyme disease between the two continents, with B. garinii associated with neurologic disease, B. afzelii associated with late skin involvement, and B. burgdorferi ss associated with arthritis.7,9 Because of the prominence of musculoskeletal manifestations with B. burgdorferi ss infection, this chapter focuses mainly on Lyme disease in North America.
Ticks and Lyme Disease
Lyme disease is found primarily in temperate climates where humans can have incidental exposure to questing ticks. Ixodes ticks have a 2-year life span in which they pass through three developmental stages—larva, nymph, and adult—feeding only once per stage.10 B. burgdorferi is not passed transovarially and is maintained by passage between reservoir hosts and ticks. Small rodents are the main reservoirs for B. burgdorferi ss and B. afzelii, whereas birds are the principal haven for B. garinii.7 In the southern United States, ticks feed preferentially on lizards, which are not competent reservoirs for B. burgdorferi; this may explain in part the rarity of Lyme disease in this region.
Larvae acquire B. burgdorferi after feeding on an infected reservoir host in early spring and then molt to nymphs, which lay dormant until the following late spring and summer. The peak incidence of Lyme disease is in the summer months, when humans come in contact with questing nymphs, which have more promiscuous feeding patterns.10 Engorged nymphs molt into adult ticks, which feed almost exclusively on deer. B. burgdorferi does not persist in deer, which serve to maintain and propagate the tick population.
Pathogenesis
Borrelia burgdorferi Invasion of the Mammalian Host
During tick feeding, B. burgdorferi migrate from the tick midgut to the salivary glands, where they are deposited with saliva into the blood meal host.11,12 Migration takes about 24 hours, during which time spirochetes multiply and undergo phenotypic changes that permit their survival in mammals. Spirochetes multiply first at the tick bite site in the skin, and if not eliminated by the cutaneous immune response, they can disseminate through tissues and the bloodstream to infect any organ system at least transiently. The degree to which B. burgdorferi cause disease in tissues depends on spirochete virulence, growth conditions that allow persistence at a particular site, and host factors that modulate the inflammatory response.
Analysis of the B. burgdorferi genome has revealed no known virulence factors common to other bacterial pathogens to help explain the pathogenesis of Lyme disease.13,14 Instead, the genome is remarkably rich in genes encoding putative lipoproteins, only a handful of which have been studied in detail. Outer surface protein (Osp) A is a midgut adhesin required for spirochete infection of ticks.15 Osp C is essential for initial infection of the mammal but is dispensable after spirochetes have disseminated and colonized other tissue.16 To do so, B. burgdorferi harnesses host plasmin to move through tissues17,18 and expresses adhesins including decorin binding proteins A and B, BBK32, and p66, which allow it to bind to extracellular matrix proteins and integrins on cells.19–22 Expression of VlsE, an Osp that undergoes antigenic variation, is required for infection to persist in immunocompetent hosts.23,24
Pathology of Lyme Disease
Because intact spirochetes are seen only rarely in tissue specimens and the spirochete genome reveals no known toxins, the pathology of Lyme disease is believed to be due to the host inflammatory response to B. burgdorferi components rather than tissue destruction by the spirochete itself. Histopathologic studies of EM lesions, cardiac tissue, synovial biopsy specimens, and limited nervous system tissue (meninges, spinal cord, and nerve roots) reveal varying degrees of monocytic and lymphoplasmacytic infiltrates, especially perivascular, that stain positively for cell surface markers for macrophages, T cells, and B cells.25,26 The joint effusions of patients with Lyme arthritis reveal acute inflammation with elevated leukocyte counts, whereas the synovium resembles that of rheumatoid arthritis, with chronic inflammation mediated by mononuclear cell infiltration and pseudolymphoid follicles formed by T cells, B cells, and plasma cells. In the synovium and less commonly the epineural area, perivascular infiltrates can be associated with endarteritis obliterans.
Immune Response to Borrelia burgdorferi
Innate immune cells respond to B. burgdorferi through engagement of the Toll-like receptor (TLR) family of pattern recognition receptors, especially TLR2/TLR1 heterodimers (lipoproteins), TLR5 (flagellin), and TLR9 (spirochete DNA).26 As a consequence, proinflammatory cytokines (including interleukin-1β [IL-1β] and tumor necrosis factor), chemokines (IL-8), nitric oxide, and prostaglandins that recruit inflammatory cells to the site of infection are produced.26–30 B. burgdorferi also induces matrix metalloproteinase expression in tissues through TLR-dependent and non–TLR-dependent pathways that contribute to pathology.30 Other pattern recognition receptors may be engaged by B. burgdorferi after its ingestion by phagocytes, including the intracellular NOD2 receptors, which respond to peptidoglycan and have been shown to potentiate the inflammatory response in vitro.31
Humoral immunity is a key host defense against B. burgdorferi infection. B. burgdorferi lipoproteins are B cell mitogens, and antibodies that arise in the absence of T cell help are sufficient to resolve inflammation and prevent challenge infection in the mouse model of Lyme borreliosis.32,33 With the induction of adaptive immunity, IgG-containing immune complexes and cryoglobulins can be found in the serum of patients with Lyme disease and are concentrated in the joints of patients who develop Lyme arthritis.34 B cell–recruiting chemokines such as CXCL13 and pathogen-specific antibody production can be found in the CSF of patients with neuroborreliosis35,36; some of these antibodies can also bind neural antigens.37–40
B. burgdorferi infection primes CD4+ and CD8+ T cells, and the predominance of T helper type 1 responses correlates with more severe arthritis and neuroborreliosis.42,43 Th17 cells are also involved, as demonstrated by the finding that the B. burgdorferi neutrophil-activing protein A (NapA) can elicit IL-17 from synovial fluid T cells ex vivo.44 There is an association between T cell and B cell responses to Osp A and the development of antibiotic-refractory Lyme arthritis.45,46 Although evidence has been presented to suggest an autoimmune etiology (see later section on antibiotic-refractory arthritis), the self-limited nature of Lyme arthritis also raises the possibility that the immune responses detected are appropriate and directed toward eliminating persisting antigens rather than viable organisms. Alternatively, prolonged arthritis may be due to abnormal or delayed regulation of the host immune response when the pathogen and its inflammatory products have been eliminated. Deficiency in CD25+ T regulatory cells prolongs murine Lyme arthritis,47 and synovial fluid γδ T cells isolated from patients with Lyme arthritis can modulate B. burgdorferi–specific CD4+ T cell responses by inducing apoptosis in a Fas-dependent fashion.48
Mechanisms of Spirochete Persistence
When visualized in vivo, B. burgdorferi resides primarily in the extracellular matrix in connective tissue.25 Despite occasional sightings of spirochetes inside cells,49 an intracellular phase of the B. burgdorferi life cycle has not been shown. B. burgdorferi employs immune evasion strategies of an extracellular pathogen, which are directed toward deterring phagocyte ingestion and antibody- and complement-mediated lysis.4 B. burgdorferi expresses Erp and complement regulator–acquiring surface proteins that bind host factor H to prevent complement-mediated lysis.50–52 To impede antibody-mediated clearance, B. burgdorferi undergoes antigenic variation23 and reduces expression of lipoproteins as infection progresses.53 The vlsE gene undergoes random rearrangement of its expression locus, producing antigenically distinct variants of VlsE, a protein essential for spirochete survival in vivo.23 In the chronic phase of B. burgdorferi infection in mice, spirochetes can be visualized in the extracellular matrix of connective tissue, especially in the skin, without an associated inflammatory response.54
Clinical Features of Lyme Disease
Lyme disease occurs in stages that reflect the immune response to the spirochete as it establishes infection in the skin and later disseminates to distant organ sites (Table 110-1). Presenting clinical manifestations depend on the stage of the illness in which patients first seek medical attention. A characteristic feature of Lyme disease is that clinical signs can resolve without specific therapy, and patients may present in later stages of the illness without exhibiting signs of early disease.
Table 110-1 Clinical Manifestations of Lyme Disease
| Early Localized Infection |
| Early Disseminated Infection |
| Late Disease |
EM, erythema migrans.
Early Localized Infection
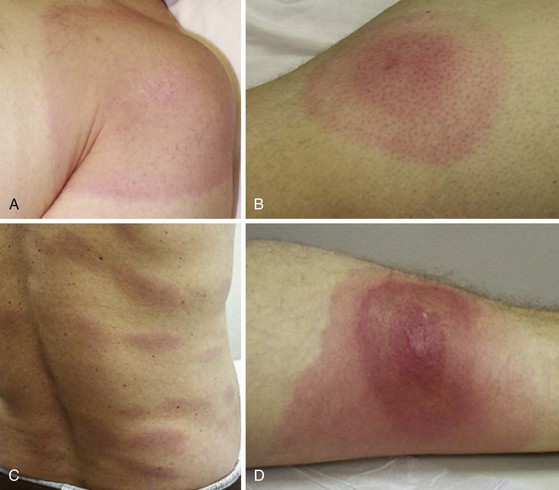
The hallmark of Lyme disease is the skin lesion EM, which is present in about 80% of patients (Figure 110-1).55 The lesion arises within 1 month (median, 7 to 10 days) at the tick bite site, especially in skin folds or where clothes bind in adults and around the hairline in children. EM begins as a red macule that expands at the rate of 2 to 3 cm/day, enlarging to more than 70 cm in diameter. Characteristic lesions greater than 5 cm in diameter in an appropriate clinical setting are sufficient for establishing the diagnosis of Lyme disease.56 EM most often manifests with uniform erythema, but central clearing can occur in larger lesions, producing a classic “bull’s eye” appearance (see Figure 110-1B). Vesicular or necrotic centers are rarer (see Figure 110-1D), but even these EM lesions have relatively few symptoms other than a tingling or burning sensation. Intense pruritus or pain is unusual and should raise concern for alternative diagnoses.
EM may be accompanied by systemic flulike symptoms including low-grade fever, malaise, neck pain or stiffness, arthralgias, and myalgias.57,58 Particularly severe systemic symptoms should alert the physician to possible co-infection with another tick-borne pathogen such as Babesia microti or Anaplasma phagocytophilum (the agent of human granulocytic anaplasmosis, formerly known as human granulocytic erhlichiosis). Lyme disease can also manifest with systemic symptoms alone.59,60 Absence of upper respiratory or gastrointestinal symptoms may help distinguish Lyme disease from common viral infections. Musculoskeletal complaints and debilitating fatigue associated with Lyme disease should be distinguished from fibromyalgia and chronic fatigue syndrome, which are typically more insidious in onset and are not associated with objective findings or laboratory abnormalities.
A newly recognized southern tick–associated rash illness (STARI) can produce a skin lesion similar to the bull’s eye form of EM.61 The rash is associated with the bite of the Lone Star tick, Amblyomma americanum, which is endemic to the southeastern and south-central states, but which also can be found as far north as Maine or west as central Texas and Oklahoma. Similar to EM, systemic symptoms can accompany the rash of STARI, but disease in organs other than the skin does not occur. The etiology of STARI is unknown. Although a noncultivable spirochete named Borrelia lonestari has been found in A. americanum, STARI patients do not develop positive Lyme serologies and the organism has not been found in skin biopsy specimens of the STARI lesions.62 Antibiotics resolve EM and STARI, but STARI patients recover more quickly from systemic symptoms than do patients with EM.
Skin Disease
Fifty percent of patients with untreated Lyme disease develop multiple EM lesions, a sign of disseminated infection (see Figure 110-1C).55 Secondary lesions are typically smaller and can occur anywhere on the body, but they are most noticeable on the trunk. The lesions usually appear as flat macules and can develop partial central clearing. EM lesions may be accompanied by migratory muscle, joint, and periarticular pain that lasts hours to days, but frank arthritis is now considered a late manifestation of the disease.
Cardiac Disease
The incidence of cardiac involvement has declined to 1% in recent years, possibly owing to earlier recognition and treatment of B. burgdorferi infection. It most often occurs within the first 2 months of infection and manifests as varying degrees of atrioventricular block, occasionally accompanied by mild myopericarditis.63 Electrophysiologic studies have mapped the conduction defect to the area above the bundle of His and involving the atrioventricular node, although multiple levels can be affected. Overt congestive heart failure is rare, and chronic cardiomyopathy, reported in Europe, has not been documented to occur in the United States.64 Patients with Lyme carditis often have a history of EM and may have concomitant arthralgia and myalgia at the time of presentation. Absence of valvular heart disease helps distinguish Lyme carditis from acute rheumatic fever, and prominent myocardial dysfunction or pericardial involvement should suggest other infectious etiologies.
Nervous System Involvement
Acute neurologic Lyme disease occurs in less than 10% of patients and most commonly manifests as cranial nerve palsy or meningitis, although radiculopathy and encephalomyelitis are also occasionally seen.65–69 Cranial palsy occurs in 8% of cases and usually affects the seventh nerve, resulting in unilateral or bilateral facial palsy. Even in endemic areas, however, onset of seventh nerve palsy in the nonwinter months is due to B. burgdorferi infection in only 25% of cases.70 Bilateral facial palsy is seen in only a few other conditions—Guillain-Barré syndrome, human immunodeficiency virus infection, sarcoidosis, and other causes of chronic meningitis—all of which are readily distinguished from Lyme disease. Rarely, other cranial nerves (III, IV, V, VI, or VIII) may be involved. Lyme meningitis manifests with fever, headache, and stiff neck similar to viral meningitis, along with a CSF lymphocytosis and elevated protein.68 In children, meningitis may occur with EM, cranial nerve involvement, and increased intracranial pressure (papilledema), which is rare in adults.68,69,71 Lyme radiculopathy typically manifests as pain, weakness, numbness, and reflex loss in a dermatomal distribution, resembling mechanical radiculopathies.67 Lyme disease should be considered when there is no obvious precipitating factor for disk-related symptoms, and imaging studies do not delineate pathology at the appropriate root level. Untreated Lyme radiculopathy can progress to become bilateral, which helps distinguish it from mechanical disease. When truncal involvement causes unilateral chest or abdominal pain, Lyme radiculopathy is often mistaken for visceral disease or early herpes zoster before the development of vesicular lesions.
Other Organ System Involvement
A variety of other organs can exhibit pathology with disseminated B. burgdorferi infection including the eye (keratitis), the ear (sensorineural hearing loss), the liver (hepatitis), the spleen (necrosis), skeletal muscle (myositis), and subcutaneous tissue (panniculitis).72 In general, other, more classic manifestations of Lyme disease are present concurrently or have been present in the recent past to suggest the diagnosis.
Late Disease
Late Neurologic Disease
Late neurologic Lyme disease is now rare; patients may present with encephalomyelitis, peripheral neuropathy, or encephalopathy.73,74 Encephalomyelitis, seen predominantly in Europe with B. garinii infection, is a slowly progressive, unifocal or multifocal inflammatory disease of the central nervous system, with increased T2 signals in the white matter on magnetic resonance imaging (MRI). CSF examination often reveals a lymphocytic pleocytosis, elevated protein, and normal glucose, and serum IgG to B. burgdorferi and intrathecal antibody production can be found. These findings help distinguish Lyme encephalomyelitis from multiple sclerosis, which may rarely be associated with positive IgG reactivity to B. burgdorferi in serum and CSF samples, but there is no intrathecal antibody production.75,76 Multiple sclerosis patients with positive Lyme serologies do not respond to antibiotics used for neurologic Lyme disease.
Late peripheral nervous system involvement manifests as a mild sensorimotor neuropathy in a “stocking and glove” distribution, with evidence of a mild confluent mononeuritis multiplex on electrophysiologic studies.77 Patients may have intermittent limb paresthesias and occasionally radicular pain. The most common finding on physical examination is reduced vibratory sensation in the lower extremities. Serum IgG to B. burgdorferi should be present, but CSF examination is normal, consistent with disease confined to the peripheral nervous system. Patients with this form of neuropathy should be evaluated for other infectious diseases (syphilis, human immunodeficiency virus, and hepatitis C virus); metabolic disorders (especially vitamin B12 deficiency, diabetes mellitus, and thyroid disease); and autoimmune diseases (antinuclear antibody [ANA] or rheumatoid factor associated).
Patients with Lyme encephalopathy complain of memory impairment and cognitive dysfunction that are best shown by formal neuropsychological testing.78,79 Occasionally, patients may have CSF abnormalities with elevated protein, lymphocytic pleocytosis, and intrathecal antibody to B. burgdorferi, but CSF examination can also be normal. Serum IgG to B. burgdorferi should be present, however, to consider the diagnosis. The mild cognitive dysfunction seen in patients with Lyme encephalopathy must be distinguished from neurocognitive deficits secondary to chronic stress, sleep deprivation, fibromyalgia, chronic fatigue syndrome, or aging. As for any chronic encephalopathy, toxic-metabolic causes should be excluded. Brain imaging studies are generally normal or show only nonspecific abnormalities and are not useful in establishing a diagnosis of encephalopathy associated with Lyme disease.
Late Skin Disease
The late skin lesion acrodermatitis chronica atrophicans is found mainly in Europe because of its association with B. afzelii infection, although any B. burgdorferi species can cause the lesion.80 Acrodermatitis chronica atrophicans develops insidiously over years and is most often found on the dorsum of the hands or feet.81 It begins as a unilateral bluish red discoloration and swelling, which evolves to atrophic, cellophane-like skin with prominent appearance of the blood vessels. About 60% of patients also have a peripheral sensory neuropathy affecting the involved extremity. A prominent lymphoplasmacytic infiltrate is shown on the skin biopsy specimen. Antibiotics can lead to improvement in pain and swelling, but atrophic skin remains.
Lyme Arthritis and Other Musculoskeletal Manifestations of Lyme Disease
Musculoskeletal symptoms are common in all stages of Lyme disease and include migratory pain in joints, tendons, bursae, and muscles.82 Typically, musculoskeletal pain affects one or two sites at a time, lasts only hours to a few days at any one location, and is associated with significant fatigue. The incidence of frank arthritis has declined from 50% in early studies to less than 10% in more recent years.26 ELISA and IgG immunoblot for B. burgdorferi are positive when arthritis appears, and B. burgdorferi DNA can be detected by polymerase chain reaction (PCR) in synovium and synovial fluid even though cultures are usually negative. Although Lyme arthritis can resemble pauciarticular juvenile arthritis or reactive arthritis, patients generally test negative for ANA, rheumatoid factor, and anticitrullinated protein antibodies (ACPAs) and do not have an increased frequency of HLA-B27 alleles. Joint fluid analysis and synovial histopathology cannot distinguish these entities. Axial and sacroiliac joint involvement is not a feature of Lyme disease, but enthesitis can be seen. Most patients with Lyme arthritis have positive two-tier serologic tests for B. burgdorferi infection.
Arthritis usually begins months or years after B. burgdorferi infection and is predated by migratory arthralgias in half of patients.82 The most typical pattern is a monoarticular or oligoarticular arthritis involving one or a few large joints (fewer than five total), with the knee affected in 80% of cases. Joints are warm with large effusions, often greater than 100 mL in the knee, but comparatively little pain. Synovial fluid is inflammatory with white blood cell counts ranging from approximately 2000 to 70,000/mm3 (median ≈ 24,000/mm3), with a predominance of neutrophils.83 Depending on the chronicity of the arthritis, synovial biopsy specimens reveal only mononuclear cell infiltration or more advanced changes consistent with rheumatoid synovium.25 Large effusions can lead to Baker’s cyst formation and rupture. The temporomandibular joint is also frequently involved and in one study was the first joint to be affected in 25% of patients with arthritis.82 Other joints commonly affected include the shoulder, ankle, elbow, wrist, and hip. Lyme arthritis is often intermittent, with episodes lasting a few weeks to months. Recurrent episodes are notable for smaller effusions and progressive synovial hypertrophy, bony erosion, and cartilage destruction. A small percentage (<10%) of patients with intermittent arthritis settle into a pattern of chronic arthritis, generally affecting only a single joint and often the knee. Inflammation of a single joint that persists for more than 12 months would be an unusual presenting manifestation of Lyme arthritis, as is the prominent involvement of small joints.
The natural history of Lyme arthritis suggests that it is a self-limited disorder. In the late 1970s, before the use of antibiotics for Lyme disease, 21 patients who presented with EM and later developed Lyme arthritis were followed for 1 to 8 years without antimicrobial therapy.82 Six patients had only a single episode of arthritis, and the remaining 15 had recurrent episodes that decreased in frequency over the study period. On average, the number of patients who continued to experience episodes of arthritis decreased by 10% to 20% each year. Similar results were found in children in whom antibiotic treatment for arthritis was delayed 4 years.84
Antibiotic-Refractory Lyme Arthritis
A few patients treated with standard antibiotic regimens for Lyme arthritis have persistent joint inflammation and proliferative synovitis that does not respond to further antimicrobial therapy.26,85 The pathogenesis of “antibiotic-refractory” Lyme arthritis is unknown but may be due to persistent spirochetes or their antigens, infection-induced autoimmunity, or inadequate regulation of the inflammatory response.25 Spirochete virulence may play a role as a retrospective study of archived tissue samples obtained before treatment revealed that patients who went on to develop antibiotic-refractory arthritis had a higher prevalence of infection with the more invasive RST1 B. burgdorferi strains.86,87 Although patients with antibiotic-refractory Lyme arthritis no longer have PCR evidence for spirochete DNA in tissues,85 experiments in the mouse model of Lyme borreliosis suggest that spirochete debris, including B. burgdorferi DNA, can persist near cartilage and in the entheses for extended periods after infectious spirochetes have been killed with antibiotics, particularly when the initial pathogen burden was high.88 Recently, a single nucleotide polymorphism in TLR1 (TLR1 1805 GG) that impairs the innate immune response to B. burgdorferi was found to be more prevalent among patients who developed antibiotic-refractory Lyme arthritis.89 A genetic predisposition had been suggested in earlier studies that found an increased frequency of the rheumatoid arthritis–related alleles HLA-DRB1*0401, HLA-DRB1*0101, and HLA-DRB1*0404 in patients with antibiotic-refractory Lyme arthritis.90 Because of the high prevalence of B cell and T cell responses to B. burgdorferi Osp A in patients with antibiotic-refractory arthritis, it had been proposed that immune responses to Osp A triggered by infection may be perpetuated by a self-antigen after the pathogen has been eliminated.26,90 An Osp A peptide corresponding to amino acids 163 through 175 (Osp A163-175) was found to share an epitope with human leukocyte function–associated antigen 1α, an adhesion molecule expressed on inflamed tissues.91 The leukocyte function–associated antigen 1α peptide stimulated Osp A163-175-specific T cells only weakly, however, and did not promote production of the T helper (Th)1 cytokine interferon-γ normally found in antibiotic-refractory arthritis.92 Antibodies to cytokeratin 10, a constituent of synovial capillaries, have been found in the blood and synovial tissue of patients with antibiotic-refractory Lyme arthritis.93 These antibodies also react with Osp A and may contribute to ongoing inflammation when infection is cleared. Linked T and B cell responses to peptides of epidermal cell growth factor have been found in about 50% of patients with antibiotic-refractory Lyme arthritis, but their role in perpetuating the inflammatory response is unclear.94 If autoimmunity is responsible for antibiotic-refractory Lyme arthritis, it must eventually succumb to immune regulation because even this form of Lyme arthritis generally resolves within 4 to 5 years.82,85 In this regard, the presence of a higher percentage of T regulatory cells correlates with more rapid resolution of joint inflammation after treatment for antibiotic-refractory Lyme arthritis.95
Diagnosis
The diagnosis of Lyme disease should be considered in individuals who present with an appropriate clinical history and who have a reasonable risk of exposure to B. burgdorferi–infected ticks (Figure 110-2).36 Supporting serologic evidence is necessary to secure the diagnosis for all stages of infection except for early localized disease in which EM can be recognized by morphologic features alone. Routine laboratory tests are nonspecific, with some patients exhibiting mildly elevated white blood cell (neutrophil) counts, erythrocyte sedimentation rates, and liver function tests. Culture or microscopic visualization of spirochetes in clinical samples is not sensitive enough for routine use in diagnosis. Culture of a skin biopsy specimen taken from the leading margin of an EM lesion is an exception, with B. burgdorferi detected in more than 40% of samples, but is rarely necessary to identify EM.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree