47 Low Back Pain
More than 90% of these patients are largely pain free within 8 weeks.
Psychosocial and other factors that predict risk of chronic disabling LBP should be assessed.
The major indication for back surgery is presence of a serious or progressive neurologic deficit.
Epidemiology
An estimated 65% to 80%1 of the population will experience LBP during their lifetime. LBP is the most prevalent chronic pain syndrome and the leading cause of limitation of activity in patients younger than the age of 45. It is also the second most frequent reason for a visit to the physician’s office and the third most common surgical indication.2 The incidence of LBP increases with age, and LBP more commonly affects women.
The natural history of back pain, especially the duration and chronicity, remains somewhat controversial. Regardless, studies show that pain and function improve substantially in most patients within 1 month,3 and more than 90% are better at 8 weeks.4 These patients, however, remain susceptible to future relapses that also tend to be brief. The remaining 7% to 10% develop chronic LBP and it is these individuals who are largely responsible for the high costs associated with LBP. The most recent reliable data, from 1998, estimated the direct cost of managing LBP in the United States to be $90 billion5 with substantial additional indirect costs due to lost time from work and decreased productivity.
A number of risk factors have been associated with LBP including heredity, psychosocial factors, heavy lifting, obesity, pregnancy, weaker trunk strength, and cigarette smoking.6 Persistence of disabling LBP has been associated with the presence of maladaptive pain coping behavior, nonorganic signs, functional impairment, poor general health status, and psychiatric comorbidities.7
Anatomy
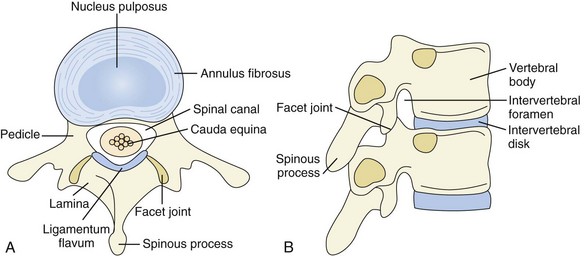
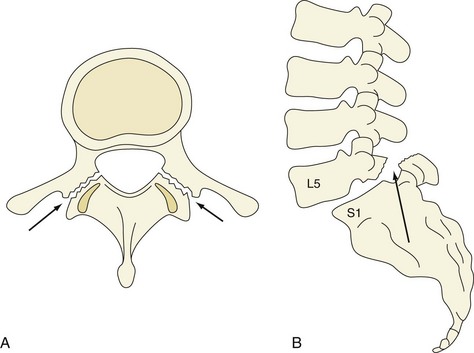
The lumbar spine is composed of five vertebrae. Each vertebra consists of a body anteriorly and a neural arch that encloses the spinal canal posteriorly (Figure 47-1). Their cartilaginous end plates cover the superior and inferior surfaces of the vertebral body.
History
The major focus in the initial evaluation of a patient with LBP is to identify the small fraction8 (<5%) of patients who may have neural compression or underlying systemic disease (infection, malignancy, or spondyloarthritis) as the cause of back pain. These patients require early diagnostic testing and may require specific treatment (e.g., antibiotics for vertebral osteomyelitis) or urgent treatment (e.g., surgical decompression in a patient with major or progressive neural compression). As such, clues to the presence of underlying systemic disease9–11 (Table 47-1), often referred to as “red flags,” should be carefully sought. It is also important to look for any social or psychologic distress such as job dissatisfaction, pursuit of disability compensation, and depression that may amplify or prolong the pain.11
Table 47-1 Red Flags for Potentially Serious Underlying Causes of Low Back Pain
| Spinal Fracture |
| Infection or Cancer |
| Cauda Equina Syndrome |
| Spondyloarthritis |
Mechanical LBP is due to an anatomic or functional abnormality in the spine that is not associated with inflammatory or neoplastic disease. It typically increases with physical activity and upright posture and tends to be relieved by rest and recumbency. More than 95% of LBP is mechanical,8 and degenerative change in the lumbar spine is the most common cause of mechanical LBP.9 Severe and acute mechanical LBP in a postmenopausal woman would be suspicious for a vertebral compression fracture secondary to osteoporosis. Nocturnal pain suggests the possibility of underlying infection or neoplasm as the cause of LBP.
Inflammatory LBP,12 as seen in the spondyloarthritides, is more common in men younger than 40 years of age. It is associated with marked morning stiffness that usually lasts for more than 30 minutes. The pain frequently improves with exercise but not with rest. Pain is often worse during the second half of the night, and some patients complain of alternating buttock pain.
It is important to ask the patient if the back pain radiates into the lower extremities, suggesting pseudoclaudication (neurogenic claudication) secondary to spinal stenosis or sciatica (usually secondary to a herniated disk). Young adults are more likely to experience disk herniations, and elderly patients are more likely to have spinal stenosis. Sciatica results from nerve root compression and produces pain in a dermatomal (radicular) distribution, usually to the level of the foot or ankle. The pain is lancinating, shooting, and sharp in quality. It is frequently accompanied by numbness and tingling and may be accompanied by sensory and motor deficits. Sciatica due to disk herniation typically increases with cough, sneezing, or the Valsalva maneuver. Sciatica should be differentiated from non-neurogenic sclerotomal pain. This pain can arise from pathology within the disk, facet joint, or lumbar paraspinal muscles and ligaments. Like sciatica, sclerotomal pain is often referred into the lower extremities, but unlike sciatica, sclerotomal pain is nondermatomal in distribution, it is dull in quality, and the pain usually does not radiate below the knee or have associated paresthesias. Most radiant pain is sclerotomal.9 Bowel or bladder dysfunction should suggest the possibility of the cauda equina syndrome.
Physical Examination
A physical examination usually does not lead to a specific diagnosis. Nevertheless, a general physical examination including a careful neurologic examination may help identify those few but critically important cases of LBP that are secondary to a systemic disease or have clinically significant neurologic involvement (see Table 47-1).
Limited spinal motion (flexion, extension, lateral bending, and rotation) is not associated with any specific diagnosis because LBP due to any cause may limit motion. Range of motion measurements, however, can help in monitoring treatment.6 Chest expansion of less than 2.5 cm has specificity but not sensitivity for ankylosing spondylitis.12
In patients with a history of LBP that radiates into the lower extremities (sciatica, pseudoclaudication, or referred sclerotomal pain) a straight leg–raising test should be performed. With the patient lying on his or her back, the heel is placed in the palm of the examiner’s hand. With the knee fully extended the leg is raised progressively. This places tension on the sciatic nerve (that takes origin from L4, L5, S1, S2, and S3) and thereby stretches the nerve roots (especially L5, S1, and S2). If any of these nerve roots is already irritated, such as by impingement from a herniated disk, further tension on the nerve root by straight leg–raising will result in radicular pain that extends below the knee. The test is positive if radicular pain is produced when the leg is raised less than 70 degrees. Dorsiflexion of the ankle further stretches the sciatic nerve and increases the sensitivity of the test. Pain experienced in the posterior thigh or knee during straight leg–raising is generally from hamstring tightness and does not represent a positive test. The straight leg–raising test is sensitive but not specific for clinically significant disk herniation at the L4-5 or L5-S1 level (the sites of 95% of clinically meaningful disk herniations). False-negative tests are more frequently seen with herniation above the L4-5 level. The straight leg–raising test is usually negative in patients with spinal stenosis. The crossed straight leg–raising test (with sciatica reproduced when the opposite leg is raised) is highly specific but insensitive for a clinically significant disk herniation.6,11,13,14
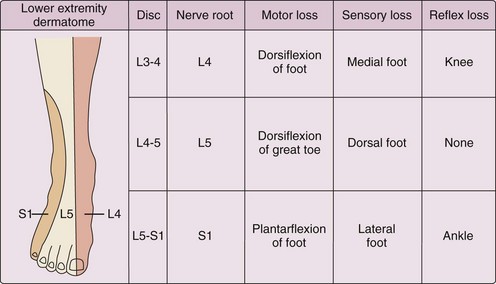
The neurologic evaluation (Figure 47-2) of the lower extremities in a patient with sciatica can identify the specific nerve root involved. The evaluation should include motor testing with focus on dorsiflexion of the foot (L4), great toe dorsiflexion (L5), and foot plantar flexion (S1); determination of knee (L4) and ankle (S1) deep tendon reflexes; and tests for dermatomal sensory loss. The inability to toe walk (mostly S1) and heel walk (mostly L5) indicate muscle weakness. Muscle atrophy can be detected by circumferential measurements of the calf and thigh at the same level bilaterally.6
Patients involved with litigation or with psychologic distress occasionally exaggerate their symptoms. They may display nonorganic signs where the objective findings do not match the subjective complaints such as with nonanatomic motor or sensory loss. A number of tests to detect this have been described by Waddell and co-workers.15 The most reproducible tests are the presence of superficial tenderness, overreaction during the examination, and observation of a discrepancy in the straight leg–raising test done in the seated and supine positions.
Diagnostic Tests
Imaging
The major function of diagnostic testing, especially imaging, is the early identification of pathology in those few patients who have evidence of a major or progressive neurologic deficit and those in whom an underlying systemic disease is suspected (see Table 47-1). Otherwise, imaging is not required unless significant symptoms persist beyond 6 to 8 weeks. This approach avoids unnecessary early testing because more than 90% of the patients will have recovered spontaneously by 8 weeks.6,8 Furthermore, neither magnetic resonance imaging (MRI) nor plain radiographs obtained early in the course of LBP evaluation improves clinical outcome, predicts recovery course, or reduces the overall cost of care.2,16
A significant problem with all imaging studies is that many of the anatomic abnormalities identified in patients with LBP are also commonly present in asymptomatic individuals and are frequently unrelated to the back pain.9 Often these abnormalities result from age-related degenerative changes, which begin to appear even in early adulthood and are among the earliest degenerative changes in the body.17 Although clinically challenging and sometimes impossible, one should refrain from making causal inferences based solely on imaging abnormalities in the absence of corresponding clinical findings because this may lead to unnecessary, invasive, and costly interventions.
Given the weak association between imaging abnormalities and symptoms, it is not surprising that in up to 85% of patients a precise pathoanatomic diagnosis with identification of the pain generator cannot be made.11 Patients should understand that the reason for imaging is to rule out serious conditions and that common degenerative findings are expected. Ill-considered attempts to make a diagnosis on the basis of imaging studies may reinforce the suspicion of serious disease, magnify the importance of nonspecific findings, and label patients with spurious diagnoses.
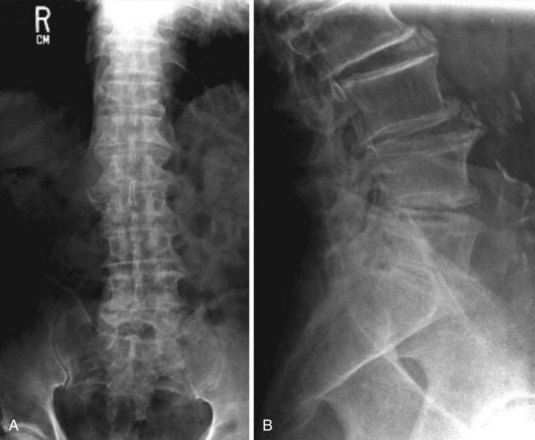
Plain radiographs and MRI are the major modalities used in the evaluation of patients with LBP. In patients with persistent LBP of greater than 6 to 8 weeks’ duration despite standard therapies, radiography may be a reasonable first option if there are no symptoms suggesting radiculopathy or spinal stenosis.18 Anteroposterior and lateral views are usually adequate. Oblique views substantially increase radiation exposure and add little new diagnostic information. Gonadal radiation in a woman from a two-view radiograph of the lumbar spine is equivalent to radiation exposure from a chest radiograph taken daily for more than 1 year.18
Abnormalities on radiography such as single-disk degeneration, facet joint degeneration, Schmorl’s nodes (protrusion of the nucleus pulposus into the spongiosa of a vertebra), spondylolysis, mild spondylolisthesis, transitional vertebrae (the “lumbarization” of S1 or “sacralization” of L5), spina bifida occulta, and mild scoliosis are equally prevalent in individuals with and without LBP.8,9,19
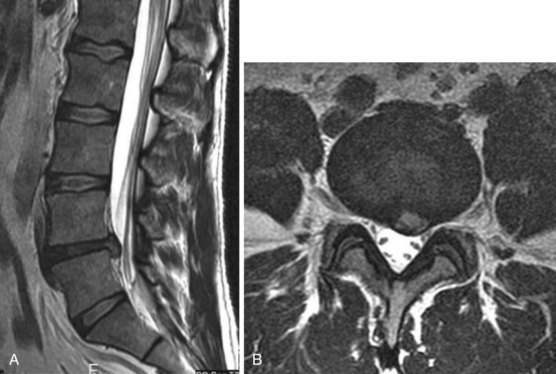
MRI without contrast is generally the best initial test for patients with LBP who require advanced imaging. It is the preferred modality for the detection of spinal infection and cancers, herniated disks, and spinal stenosis.8 MRI testing for LBP should largely be limited to patients in whom there is a suspicion of systemic disease (such as infection or malignancy), for the preoperative evaluation of patients who are surgical candidates on clinical grounds11,18 (e.g., the presence of a significant or progressive neurologic deficit), or for those patients with radiculopathy or spinal stenosis who are candidates for epidural corticosteroids.18 Disk abnormalities are commonly noted on MRI studies but often have little or no relationship with the patient’s symptoms. A disk bulge is a symmetric, circumferential extension of disk material beyond the interspace. A disk herniation is a focal or asymmetric extension. Herniations are subdivided into protrusions and extrusions. Protrusions are broad-based, whereas extrusions have a “neck” so that the base is narrower than the extruded material. Bulges (52%) and protrusions (27%) are common in asymptomatic adults, but extrusions are rare.8 MRI with the intravenous contrast agent, gadolinium, may be useful for the evaluation of patients with prior back surgery (with no hardware present) to help in the differentiation of scar tissue from recurrent disk herniation.
Electrodiagnostic Studies
Electrodiagnostic studies can be helpful in the evaluation of some patients with lumbosacral radiculopathy. The main procedures are electromyography and nerve conduction studies. These studies can confirm nerve root compression and define the distribution and severity of involvement. Whereas studies such as MRI can only provide anatomic information, electrodiagnostic studies provide physiologic information that may support or refute the findings on imaging. Electrodiagnostic testing is therefore mostly considered in patients with persistent disabling symptoms of radiculopathy where there is discordance between the clinical presentation and findings on imaging. Electromyography and nerve conduction studies can also be helpful in differentiating the limb pain of peroneal nerve palsy or lumbosacral plexopathy from that of L5 radiculopathy. These studies are also useful in evaluating possible factitious weakness. Electrodiagnosis is unnecessary in a patient with an obvious radiculopathy. It should be noted that electromyographic changes depend on the development of muscle denervation following nerve injury and may not be detected for 2 to 3 weeks after the injury. Another limitation is that electromyographic abnormalities may persist for over a year following decompressive surgery.20
Differential Diagnosis
LBP usually originates from pathology within the lumbar spine or associated muscles and ligaments (Table 47-2). Rarely pain is referred to the back from visceral disease. In the vast majority of patients with LBP, the pain is mechanical.11 Degenerative change in the lumbar spine is the largest contributor to the mechanical causes of LBP8 (see Table 47-2) and indeed the most commonly identified cause of back pain.
Table 47-2 Causes of Low Back Pain
| Mechanical |
| Neoplastic |
| Inflammatory |
| Infectious |
| Metabolic |
| Referred Pain to Spine |
* Related to degenerative changes.
Lumbar Spondylosis
The current common usage of the term lumbar spondylosis incorporates degenerative changes in both the anteriorly placed discovertebral joints and the posterolaterally placed facet joints.6 These degenerative or osteoarthritic changes are seen radiographically as disk or joint space narrowing, subchondral sclerosis, and osteophytosis (Figure 47-3).
Imaging evidence of lumbar spondylosis is common in the general population, increases with age, and may be unrelated to back symptoms. Radiographic abnormalities such as single disk degeneration, facet joint degeneration, Schmorl’s nodes, mild spondylolisthesis, and mild scoliosis are equally prevalent in persons with and without back pain.22 The situation is further complicated by the observation that patients with severe mechanical LBP may have minimal radiographic changes, and conversely patients with advanced changes may be asymptomatic.
The terms internal disk disruption and diskogenic low back pain are used interchangeably and remain controversial diagnoses.13 The disorder is diagnosed by provocative diskography. Following contrast injection into several disks in sequence, the radiographic appearance and induced pain at each level are assessed. If injection into a disk reproduces a patient’s usual LBP, the test is considered positive. Advocates of this technique interpret a positive diskogram as defining the particular disk as the primary pain generator, and spinal fusion or disk arthroplasty is frequently recommended.2 However, injection into a disk can simulate the quality and location of pain known not to originate from that disk.23 Furthermore, diskographic abnormalities and induced pain are frequently seen in asymptomatic persons and, more importantly, the diskogenic pain attributed to disk disruption frequently improves spontaneously.8,11 Therefore the clinical importance and appropriate management of this condition remains unclear.
Focal high signal in the posterior annulus fibrosus as seen on T2-weighted MRI images, sometimes referred to as a high-intensity zone, is believed to represent tears in the annulus fibrosus and to correlate with positive findings on provocative diskography.8 The high prevalence of high-intensity zones in asymptomatic individuals limits its clinical value.24
Disk Herniation
Intervertebral disk herniation occurs when the nucleus pulposus in a degenerated disk prolapses and pushes out the weakened annulus, usually posterolaterally. Imaging evidence of disk herniation has a high prevalence in the general population with one study finding MRI evidence of disk herniation in 27% of asymptomatic individuals.25 Occasionally, however, the herniated disk can cause nerve root impingement leading to lumbosacral radiculopathy (Figures 47-4 and 47-5). A herniated intervertebral disk is the most common cause of sciatica.8
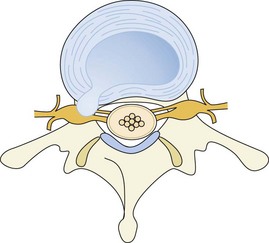
Figure 47-4 Schematic drawing showing posterolateral disk herniation resulting in nerve root impingement.
The lumbosacral spine is susceptible to disk herniation because of its mobility. Seventy-five percent of flexion and extension occurs at the lumbosacral joint (L5-S1), and 20% occurs at L4-526 (with more torsion at the L4-5 level). Probably related to this, 90% to 95% of clinically significant compressive radiculopathies occur at these two levels.11
Disk herniation is rare in young individuals with the frequency increasing with age. The peak frequency of herniation at the L5-S1 and L4-L5 levels is between the ages of 44 and 50 with a progressive decline in frequency thereafter.27
The clinical features of disk herniation resulting in lumbosacral radiculopathy have already been discussed (see history, physical examination, and Figure 47-2). It should be noted that immediate imaging is unnecessary in patients without a clinically significant neurologic deficit and no red flags to suggest an underlying systemic pathology (see Table 47-1). L1 radiculopathy is rare and presents with symptoms of pain, paresthesias, and sensory loss in the inguinal region.28 L2, L3, and L4 radiculopathies are uncommon and more likely to be seen in older patients with lumbar spinal stenosis.
The natural history of disk herniation is favorable with progressive improvement expected in most patients. Sequential MRI studies reveal that the herniated portion of the disk regresses with time and there is partial or complete resolution in two thirds of cases after 6 months.11,29 Only approximately 10% of patients have sufficient pain after 6 weeks of conservative care, and for this group decompressive surgery is considered.11 Even a sequestered fragment (piece of herniated material that breaks off and is free in the epidural space) tends to be reabsorbed with time.30
Rarely a large midline disk herniation, usually L4-5,9 compresses the cauda equina resulting in cauda equina syndrome. Patients usually present with LBP, bilateral radicular pain, and bilateral motor deficits with leg weakness. Physical examination findings are often asymmetric. Sensory loss in the perineum (saddle anesthesia) is common, and urinary retention with overflow incontinence is usually present.11 Fecal incontinence may also occur. Other causes of cauda equina syndrome include neoplasia, epidural abscess, hematoma, and rarely lumbar spinal stenosis. Cauda equina syndrome is a surgical emergency because neurologic results are affected by the time to decompression.6
Spondylolisthesis
Isthmic spondylolisthesis (Figure 47-6) is caused by bilateral spondylolysis. Spondylolysis is a defect in the pars interarticularis that is most commonly seen at L5. It is typically a fatigue fracture acquired early in life that is more commonly seen in boys. Spondylolysis progresses to spondylolisthesis in approximately 15% of patients.31
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree