10
Genitourinary and Gynecological Systems
At the completion of this chapter the reader should be able to do the following:
1. Name common genitourinary and gynecological disorders that athletic personnel encounter in the care of the athlete
2. Describe conditions of the genitourinary and gynecological systems that warrant referral
3. Explain the physiology of ovulation and menstruation
4. Describe the physiological changes that occur in pregnancy
5. Identify the limitations of the pregnant athlete
6. Describe preventive strategies for genitourinary and gynecological disorders, and for sexually transmitted infections (STIs)
7. Refer patients with signs or symptoms of an STI to a physician
Overview of Anatomy and Physiology
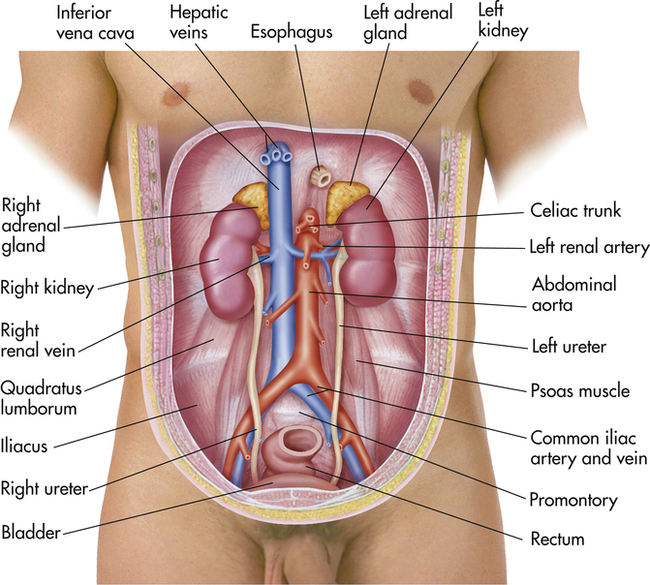
Anatomy of the Kidneys, Ureters, and Urinary Bladder
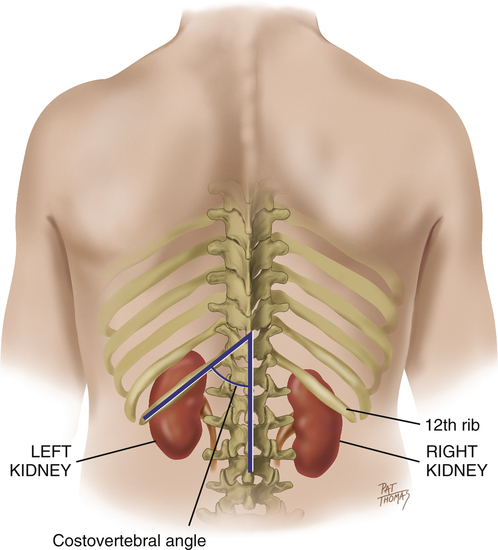
The kidneys act to remove excess water, salts, and products of metabolism from the blood in order to maintain proper acid–base status. The body’s waste products are then conveyed in the urine to the urinary bladder by the ureters. Normally, an individual has two kidneys, two ureters, and a single urinary bladder (Figure 10-1). The kidneys lie posterior to the peritoneum in the retroperitoneal space on the posterior abdominal wall, alongside the spine and against the psoas major muscles. The kidneys are bean-shaped organs whose upper poles are protected by the lower bony thorax. Because of the large size of the right lobe of the liver, the right kidney lies at a slightly lower level than the left. In muscular individuals and those with well-developed abdominal musculature, the kidneys are generally not palpable on examination.
The surrounding anatomy of the two kidneys differs anteriorly (Figure 10-2). The right kidney is associated with the liver and separated from it by the hepatorenal recess. The left kidney is associated with the left adrenal gland, stomach, spleen, pancreas, a portion of the small bowel, and the descending colon. They lie well protected posteriorly by the costovertebral angle between the twelfth rib and the vertebral spine. In addition, both kidneys are attached superiorly to the diaphragm and move slightly on respiration.
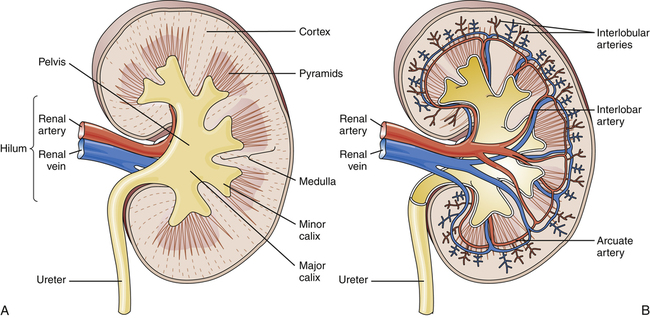
The kidneys are enclosed in a strong fibrous capsule that is surrounded by a layer of fat called perirenal fat. The unique characteristics of the density of the kidney itself and the perirenal fat allow for the kidneys to be visualized on abdominal radiographs. The kidney is a solid organ with a thick cortex under the fibrous capsule (Figure 10-3). Filtration begins at the medulla, continues interiorly in the calyx structures, and ends in the collection area before the ureter.
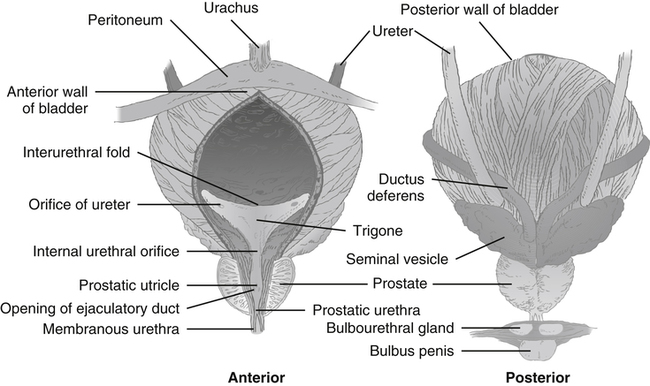
The urinary bladder is a muscular sac or vesicle that functions to store urine. Its shape, size, position, and relation to other structures vary with the amount of urine it contains. It is composed chiefly of smooth muscle. In the adult, the empty urinary bladder lies posterior to the symphysis pubis within the pelvis (Figure 10-4). As it fills, it ascends into the lower abdomen. A full bladder may reach as high as the level of the umbilicus. The ureters enter at the superolateral aspect of each side of the bladder. The bladder is then drained by a single urethra that empties from the central inferior aspect.
The blood supply to the kidneys is provided by the right and left renal arteries, respectively. These branch off from the descending aorta at nearly right angles. Venous drainage is provided by the right and left renal veins that empty into the inferior vena cava. Blood supply to the ureters is more complex, but it is principally supplied by arterial branches from the renal, aortic, common iliac, vesicular, or uterine arteries. The main arteries supplying the urinary bladder are branches of the internal iliac arteries. In the female, however, branches of the uterine and vaginal arteries also supply a portion of the blood supply to the bladder. Venous drainage occurs via the vesicular venous plexus that drains to the internal iliac vein (see Figure 10-1).
The urinary bladder is supplied by parasympathetic motor fibers to the detrusor muscle of the bladder, and sensory fibers. The sensory fibers are stimulated by stretching of the bladder, causing a sensation of fullness and activating the micturition, or urination, reflex. Micturition is preceded by contraction of the diaphragm and abdominal wall. The neck of the bladder descends, the detrusor muscle contracts by reflex, and urine is voluntarily expelled from the bladder (see Figure 10-4).
Male Genital Anatomy
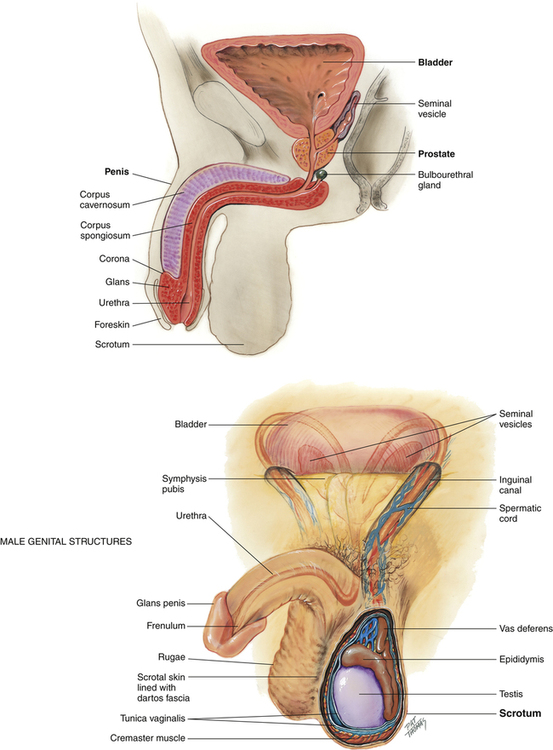
The male genital organs comprise the penis, ejaculatory duct, prostate gland, bulbourethral gland, and paired testes, each with an epididymis, ductus or vas deferens, and seminal vesicle (Figure 10-5). Spermatozoa, formed in the testes and stored in the epididymides, are contained in the semen, which is secreted by the testes and epididymides, seminal vesicles, prostate, and bulbourethral glands. The sperm, on leaving the epididymides, pass through the ductus deferens and ejaculatory ducts to reach the urethra and pass through the external urethral orifice.
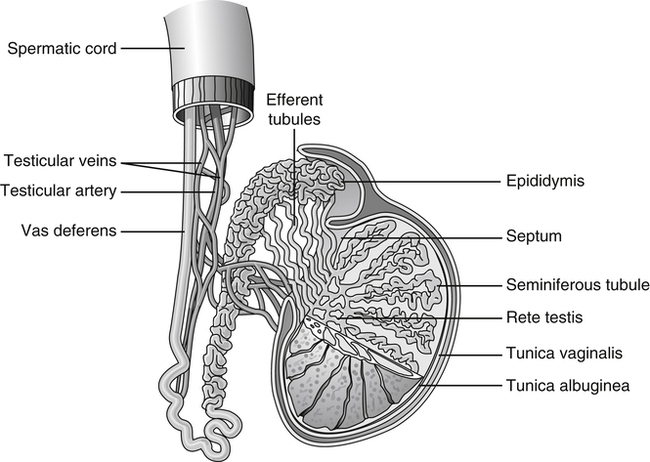
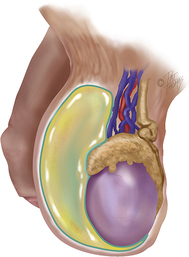
The testes are paired ovoid glands located in the scrotum and responsible for production of spermatozoa and steroid hormones. They reside away from the core of the body to maintain a slightly lower temperature of approximately 1° to 2° F below that of the body proper. The left testicle often lies slightly lower than the right testicle in the scrotum. The epididymis is associated with the posterior portion of each testicle. The testes and epididymides are covered by a dual-layered tunica vaginalis testis, which is derived prenatally from the processus vaginalis of the peritoneum (Figure 10-6). The potential cavity between these two layers or some part of the processus vaginalis may become distended with fluid, forming a hydrocele.
Female Genital Anatomy
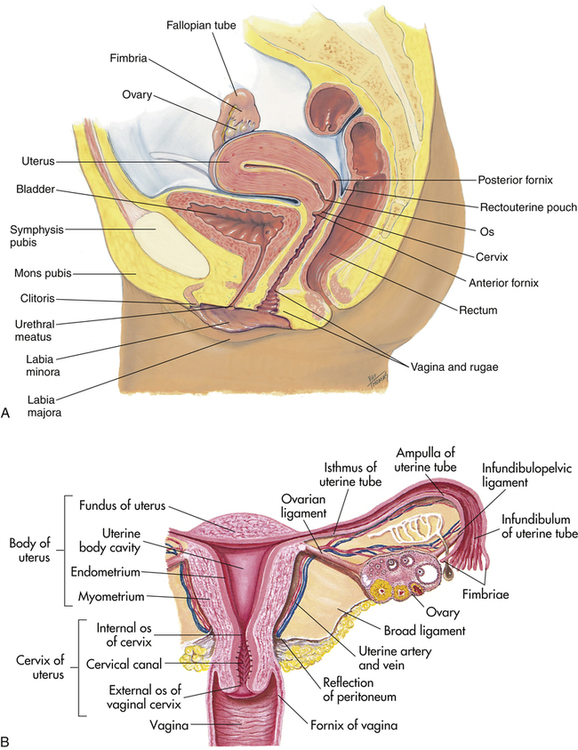
The female genital organs comprise the ovaries, fallopian tubes, uterus, vagina, and external genitalia, specifically the mons pubis, labia majora and minora, vestibule of the vagina, bulb of the vestibule, vestibular glands, and clitoris (Figure 10-7, A).
The uterus is a muscular organ that lies within the pelvis (Figure 10-7, B). The uterus functions to accept the fertilized egg and allow for implantation and development of the fetus. The upper uterine segment receives the fallopian tubes. The lower uterine segment terminates in the cervix, which opens to the vagina. The uterus has three distinct layers: a mucosa or endometrium, a muscular coat or myometrium, and a serosa or perimetrium.
Physiology of Ovulation and Menstruation
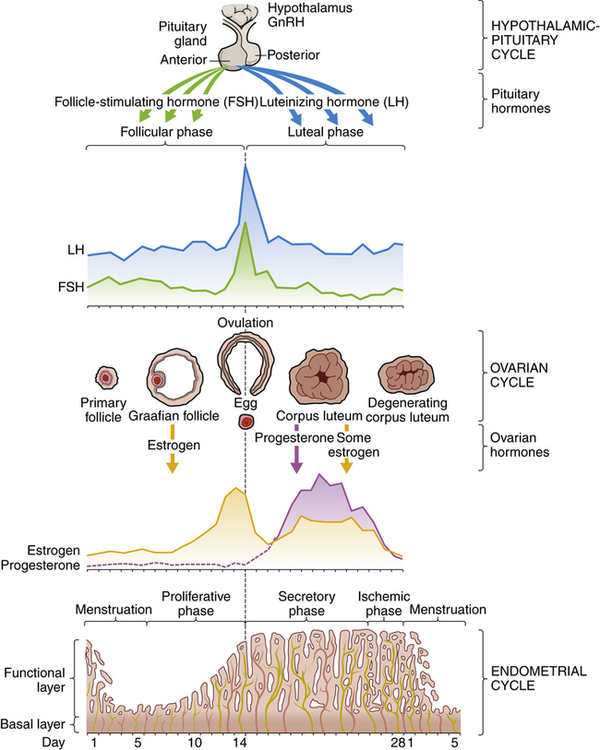
Normal menstrual cycles depend on an intact hypothalamic–pituitary axis, functioning ovaries, and a normal outflow tract. The menstrual cycle, which averages 28 days, requires a well-coordinated series of events (Figure 10-8). The normal menstrual cycle is divided into two parts: a proliferative, or follicular, phase and a secretory, or luteal, phase. During the follicular phase, estrogen and luteinizing hormone (LH) levels increase as follicle-stimulating hormone (FSH) levels decrease. The endometrium thickens during this phase. Before ovulation, estrogen sharply declines, followed by a surge in LH and a steady rise in progesterone. It is shortly after this that ovulation occurs, followed by a slight increase in core body temperature. The remnant of the follicle (i.e., corpus luteum) supplies the progesterone for the second half of the cycle. During this time, the endometrium prepares itself for implantation. If fertilization and implantation do not occur, the corpus luteum involutes and progesterone levels decline, prompting menses.
Physiological Changes of Pregnancy
Noteworthy physiological changes occur in pregnancy. Cardiac output (CO), defined as stroke volume (SV) × heart rate (HR), increases during pregnancy as a result of increases in both SV and HR. Plasma volume also increases with pregnancy.1 The high flow of blood exiting the heart can often create a benign heart murmur. Blood pressure, defined as CO × systemic vascular resistance (SVR), actually decreases because of a decrease in SVR.
Physiological responses to exercise are somewhat different in pregnancy than in the nonpregnant female.2 Respiratory rates increase with mild exercise in pregnancy compared with nonpregnant women, whereas maximal oxygen consumption (Vo2 max) is less in pregnant women compared with nonpregnant women. The respiratory quotient (Vco2/Vo2) is also increased in exercising pregnant women, suggesting that there may be a greater dependence on carbohydrates as the preferred fuel source. This may also explain the fact that hypoglycemia can develop more rapidly during prolonged strenuous exercise in pregnant athletes. In addition, the core temperature of a pregnant woman is higher than that of a nonpregnant athlete, which requires caution in the exercising expectant mother, especially in hotter climates.
Pathological Conditions of the Genitourinary System
Kidney Stones
Kidney stones, also known as renal calculi, arise in the kidney when urine becomes supersaturated with a salt that is capable of forming solid crystals. More than 5% of adults have had kidney stones.3 Renal calculi are commonly composed of calcium (75%), struvite (15%), uric acid (6%), and cystine (2%) (Box 10-1). Recurrence rates after an initial kidney stone are 14% (1 yr), 35% (5 yr), and 52% (10 yr). Males are affected approximately three times more commonly than females, and Caucasian males are affected more commonly than African-American males, although African-American males have a higher incidence of associated infection with renal calculi whereas females of all races have been noted to have a higher incidence of infected hydronephrosis. The age of onset of symptomatic renal calculi is generally in the third or fourth decades.
Signs and Symptoms
Most kidney stones originate within the kidney and proceed distally, creating various degrees of urinary obstruction as they become lodged in the narrow canal areas. Acute passage of a kidney stone from the kidney through the ureter gives rise to pain so excruciating that it has been likened to that of childbirth. The location and quality of pain are related to the position of the stone within the urinary tract. The severity of pain is related to the degree of obstruction, the presence of ureteral spasm, and the presence of any associated infection. Pain is typically described as unilateral flank pain that radiates to the groin. The individual is often writhing in pain, moving about and unable to lie still. Nausea and vomiting are common. Examination demonstrates flank tenderness, costovertebral angle tenderness, and occasionally testicular pain, notably in the absence of any testicular tenderness. The abdominal examination is often normal although bowel sounds may be hypoactive because of a mild ileus (see Chapter 9). The presence of a fever raises the possibility of an infectious complication and warrants immediate referral.
Referral and Diagnostic Tests
The mainstay of diagnostic testing for kidney stones is a urinalysis. Blood is often present in the urine and may be detectable in more than 90% of symptomatic individuals, using both a urine dipstick and microscopy. Urine pH can also be helpful because a urine pH greater than 7 suggests the presence of urea-splitting organisms and struvite stones. Alternatively, a urine pH less than 5 suggests the presence of uric acid stones. The presence of pyuria (>5 white blood cells per high-power field) in a centrifuged urine specimen should prompt a careful search for an associated infection. (Normal urine values are listed in Table 3-2.) In these cases a complete blood count (CBC) and differential, serum creatinine, and urine culture are in order.
Imaging studies may also be performed and are often done to confirm the initial diagnosis. The current imaging study most often used is the noncontrast helical computerized tomography (CT) scan.4 This is a rapid test with sensitivity in the range of 95% to 100%. The principal disadvantage of CT is that indinavir stones are not well visualized by this method. Radiographs may also be obtained and may demonstrate a radiopaque stone. Radiographs are occasionally used to monitor the passage of a stone under certain circumstances. An intravenous pyelogram (IVP) may be used in the diagnosis of kidney stones but has essentially been replaced by CT. Last, ultrasound can also be used to identify stones. Although the sensitivity and specificity of ultrasound are poorer than with other imaging techniques, there is no exposure to radiation, and therefore this is an ideal imaging tool for pregnant women.
Differential Diagnosis
The differential diagnosis for kidney stones is long and often depends on what side of the body is involved, as well as on the sex and age of the individual. The list includes urinary tract infection, pyelonephritis, urinary obstruction, testicular torsion, pelvic inflammatory disease, bowel obstruction, appendicitis, cholecystitis, biliary colic, and constipation. Among those older than 60 years of age, an abdominal aortic aneurysm may also be included as a differential diagnosis.5
Treatment
The crux of treatment for the uncomplicated passage of a kidney stone is pain management and maintenance of adequate hydration. Pain management is often obtained with narcotic analgesics or nonsteroidal antiinflammatory agents, such as ketorolac (Toradol).6 An antiemetic medication also may be added when nausea is present and deters the use of oral analgesics or hydration. The forcing of oral or intravenous fluids has not been shown to alter outcome or to improve the passage of a stone; therefore the focus should remain on maintenance of hydration. A strainer is useful to filter the urine during the passage of the stone in order to collect the stone for analysis. Antibiotics are necessary in the presence of an associated infection.
Special Concerns in the Mature Athlete
Athletes 60 years of age and older with an initial presentation of a kidney stone actually may have an abdominal aortic aneurysm (AAA). In a series of 134 patients with a symptomatic AAA presenting to the emergency department, 18% had an initial misdiagnosis of a kidney stone.5
Sports Hematuria
Sports hematuria is the benign, self-limiting presence of three or more red blood cells per high-power field in a centrifuged urine specimen and is directly associated with exercise or activity. Sports hematuria is asymptomatic and has been documented to occur in both contact and noncontact sports. The degree of hematuria is believed to be related to the intensity and duration of the exercise. In most circumstances the hematuria will resolve within 72 hours of onset in athletes without any coexisting urinary tract pathology.7
The incidence of sports hematuria is estimated to be as high as 80% in swimming, lacrosse, and track and field; 55% in football and rowing; and 20% in marathon runners (Figure 10-9). These incidence levels have led to the development of several possible causes of sports hematuria (e.g., increased permeability of the glomerulus, direct or indirect trauma to the kidneys, renal ischemia, dehydration, release of a hemolyzing factor), all of which appear to be related to exercise duration and exercise intensity.8
Differential Diagnosis
The differential diagnosis includes causes of true hematuria (i.e., red blood cells in urine) and causes of a false-positive urine blood dipstick. True hematuria may result from a urinary tract infection, urethritis, interstitial nephritis, renal papillary necrosis, nephrolithiasis (i.e., renal stone), polycystic kidney disease, kidney laceration, a neoplasm arising from any structure in the urinary tract, coagulopathy, and prostatitis in males.9 Causes of a false-positive urine dipstick examination for blood include drugs (i.e., phenazopyridine, rifampin, nitrofurantoin, phenytoin), food dyes, menses, and myoglobin in the urine.
Urinary Tract Infection
Anyone can develop a UTI; however, sexually active young women are at highest risk. Several factors have been attributed to this higher risk: a short urethra; sexual activity; delays in micturition, particularly after intercourse; and the use of diaphragms and spermicides.11 Fortunately, the risk of a complicated UTI in this population is very low, yet up to 20% of young women with a UTI will develop recurrent UTIs.12
UTIs in men are less common than in women but can occur. Overall, most UTIs in men are accounted for by older men: this is attributed to risk factors such as prostatic disease, which can cause some degree of urinary obstruction, and urinary tract instrumentation. Among younger men, UTIs may occur in men who participate in anal sex, who are not circumcised, or whose sexual partner is colonized with a uropathogen.13 Catheter-associated UTIs are also known to occur.
Treatment
UTIs are treated with antibiotics. An uncomplicated UTI can be treated with antibiotics such as trimethoprim-sulfamethoxazole, ciprofloxacin, or ofloxacin for a 3-day course (see Chapter 5). Recurrent UTIs in women or UTIs in men should be treated with a 7- to 10-day course of antibiotics with antibiotic choice based on the results of the urine culture. If a woman experiences more than three UTIs in a given year, prophylactic antibiotics may be used to prevent recurrence. Studies have challenged the use after coitus of prophylactic antibiotics, given continually at a lower dose than treatment dose, for recurrent UTIs.15 Complicated UTIs require a longer course of treatment and should be treated for 10 to 14 days.
Urethritis
Infectious causes of urethritis are typically sexually transmitted and include Neisseria gonorrhoeae (GU) and nongonococcal organisms such as Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, and Trichomonas vaginalis (NGU).16 Less common infectious causes of urethritis include lymphogranuloma venereum, herpes genitalis, and syphilis and may be associated with infectious conditions such as epididymitis, orchitis, prostatitis, or UTIs. The incidence of GU is in decline. Conversely, the incidence of NGU is rising and is notably higher during the summer months. Urethritis affects males and females equally, although up to 50% of females may be asymptomatic and homosexual males are more commonly infected than heterosexuals or homosexual females. Infectious urethritis may occur in any sexually active person, but the incidence is highest among people 20 to 24 years of age.
Testicular Torsion
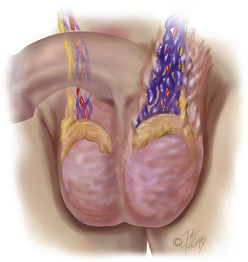
The testicle is covered by the tunica vaginalis, which attaches to the posterolateral surface of the testicle and allows for limited mobility. In the event that the testicle is able to twist or freely rotate (i.e., torsion), venous occlusion can occur, which subsequently leads to arterial ischemia causing infarction of the testicle (Figure 10-10).
The incidence of testicular torsion in males younger than 25 years is approximately 1 per 4000. The highest incidence is among males 12 to 18 years of age, with a peak incidence at age 14 years.17 Torsion predominantly affects the left testicle. A subgroup of individuals has a higher frequency of testicular torsion because of an extremely narrow attachment of the epididymis to the tunica vaginalis (i.e., bell clapper deformity).17 This attachment allows the testicle to rotate freely on the spermatic cord within the scrotal sac.18 This congenital abnormality is found in as many as 12% of males. Testicular torsion can also occur after exercise, sexual activity, or trauma, or it may develop at rest.
Signs and Symptoms
The history of testicular torsion includes the sudden onset of severe unilateral scrotal pain. The most common symptoms include scrotal swelling, abdominal pain, nausea, and vomiting.19 Less frequently a fever or urinary frequency may be documented. Examination of the scrotum reveals a tender and painful testicle that is often elevated in relationship to the contralateral testicle. The involved testicle often is in a horizontal position rather than its usual vertical orientation. The testicle may be enlarged with scrotal swelling and erythema.17 In general, elevation of the involved testicle provides no relief of pain as compared with epididymitis, in which pain relief is notable with elevation of the involved testicle.
Referral and Diagnostic Tests
Testicular torsion is a urological emergency.
The consideration of a diagnosis requires immediate and emergent evaluation by the team physician or immediate referral to an emergency department. Diagnosis and treatment within 6 hours of the onset of pain result in an 80% to 100% salvage rate for the affected testicle. Beyond this time frame, the salvage rate steadily decreases and approaches 0% at 12 hours.20
Imaging studies can provide useful information, but because testicular torsion is a clinical diagnosis, treatment should not be delayed for imaging if the diagnosis is clear. For those cases in which the diagnosis is less clear, color Doppler ultrasonography can be performed.21,22 A color Doppler is used to assess arterial blood flow to the testicle. A radionuclide scan can also be performed to assess arterial blood flow, with decreased uptake indicating a lack of blood flow to the testicle.
Treatment
Early diagnosis and referral are the keys to successful treatment. Once testicular torsion is diagnosed, a manual reduction can be attempted by the physician. Because most testicular torsion involves a “turning in” toward the midline, the process of detorsion involves rotating the affected testicle 180 degrees from medial to lateral. This rotation may need to be repeated two or three times for a complete detorsion. Success is determined by a marked decrease in pain. Detorsion can be accomplished manually in 30% to 70% of affected individuals.17 If manual detorsion is not successful, surgery is indicated for definitive treatment and involves detorsion and orchiopexy.
Prognosis and Return to Participation
The prognosis for testicular torsion depends on rapid referral and diagnosis. If detorsion is obtained within 6 hours of onset of symptoms, nearly 100% of torsive testicles can be salvaged. A delay in treatment up to 12 hours results in decreasing rates of salvage.19 Return to participation is based on the result of the torsion and physician clearance.
Hydrocele
Signs and Symptoms
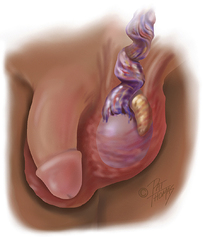
Hydroceles are usually asymptomatic. Increased fluid collections, however, can cause a scrotal aching. A hydrocele typically manifests itself as a nontender fullness in the hemiscrotum and is palpable just anterior to the testicle. Inability to clearly delineate or palpate the testicular structures or the presence of tenderness raises the possibility of an alternative diagnosis (Figure 10-11).
Referral and Diagnostic Tests
Athletic trainers should immediately refer any male with painful scrotal swelling to a physician. Although a hydrocele is not an emergency, nontender scrotal swelling that is consistent with a hydrocele needs to be examined by a physician to document its presence. An experienced physician can confirm the diagnosis of a hydrocele. An ultrasound may be performed to confirm the diagnosis in some cases. Box 10-2 lists the differential diagnoses for hydroceles.
Varicocele
A varicocele is a dilation of the pampiniform venous plexus and the internal spermatic vein within the scrotum (Figure 10-12). The etiology of a varicocele is unclear. Varicoceles occur in approximately 20% of the adult male population; however, about 40% of infertile men may have a varicocele.23
Signs and Symptoms
Approximately 80% to 90% of varicoceles occur on the left side of the scrotum because of anatomical vascular differences.23 Men are generally asymptomatic but will occasionally report an aching pain or heaviness in the scrotum. Physical examination demonstrates a soft thickening just above the testicle and has been described as feeling like a “bag of worms.” Varicoceles are staged according to size (Box 10-3).
Referral and Diagnostic Tests
The diagnosis of a varicocele is typically clear by physical examination; Valsalva’s maneuver may aid diagnosis (Figure 10-13). If the physical examination is equivocal a Doppler ultrasonogram may be performed to demonstrate the varicocele. Individuals who have a new or sudden-onset varicocele or a nonreducible varicocele in the recumbent position may warrant abdominal CT to evaluate for renal or vascular pathology as a cause of spermatic vein compression. Box 10-2 lists the differential diagnoses for varicoceles.
Treatment, Prognosis, and Return to Participation
The presence of a varicocele poses no known risks to the athlete involved in individual or team sports. After surgical correction of a varicocele, return to play is generally within 2 to 6 weeks but will depend on the specific circumstances for the athlete and the recommendations of the surgeon. Use of a protective cup is recommended for involvement in contact or collision sports if early return is allowed.23
Testicular Cancer
Testicular cancer typically affects men between the ages of 18 to 44 years. The American Cancer Society’s statistics on cancer state that in 2009, about 8400 men were diagnosed with testicular cancer, but only 380 will die of the disease.24 Hispanic men were the group who experienced the largest increase in incidence 25 whereas northern European populations were still the most affected. Asian and African populations have a low incidence of testicular cancer.26 There is increasing evidence that there may be a slight genetic component to this cancer.27 Conditions that are associated with an increased risk of testicular cancer include cryptorchidism (i.e., failure of one or both testicles to descend into the scrotum during development), maternal exposure to diethylstilbestrol (DES) while pregnant, testicular atrophy, and some possible environmental and drug exposures.28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree