19
Working with Special Populations
At the completion of this chapter the reader should be able to do the following:
1. Discuss components of the general medical history necessary when assessing persons with selected disabilities
2. Recognize the importance of the preparticipation physical examination in identifying baseline norms in the athlete with a disability
3. Identify typical symptoms and clinical signs of pathological conditions seen in athletes with selected disabilities
4. Apply typical treatment and prevention measures of pathological conditions seen in persons with selected disabilities
5. Identify the interaction of disability-related attributes with illness-related characteristics
6. Communicate effectively with athletes with selected disabilities
Introduction
Participation in organized sports and recreation by athletes with disabilities has increased significantly, with more than 3 million individuals participating in the United States alone. This has created a deeper field of competition, requiring athletes to train and compete at higher levels to attain success. Athletes in wheelchairs have long broken the 4-minute mile and have completed 26-mile marathons in less than 90 minutes. Oscar Pistorius, nicknamed “the fastest man on no legs,” has run the 100 m on two prosthetic legs with a time of 10.91 seconds. War has had an effect on sports and recreation participation by persons with disabilities. Fifteen military veterans and one active-duty service member representing the United States participated in the 2008 Paralympic Games in Beijing, China. Many more retired veterans participated.1
In the United States, special education programs make school attendance possible for more than 5 million children with disabilities.2 Adapted physical education provides opportunities to participate in sports and recreation through physical conditioning training, noncompetitive and competitive sports, outdoor physical activities, and health centers. The need for quality sports medicine care in special populations is as important as it is in the able-bodied population.
Although risks of injury and illness are inherent in all physical activity and injury rates for athletes with disabilities have generally been found to be within the same range as for athletes without disabilities,3,4 athletes with disabilities do pose some unique concerns related to the general medical assessment. Musculoskeletal injuries accounted for 79.7% of the reported injuries to athletes with disabilities, and general medical problems (i.e., illness or disability-related) accounted for 20.3% of those athletes who were unable to participate.3 Athletic trainers working with world class athletes will note that Paralympians file more therapeutic use exemptions with the governing bodies than do their Olympic counterparts; this is due to the more complex nature of their medical conditions.
Preparticipation Examination for Athletes with Disabilities
Athletes must complete a thorough preparticipation physical examination (PPE) so that baseline norms can be established. The general approach of the PPE for athletes with disabilities is similar to that for athletes without disabilities (see Figure 1-8). However, often the focus is on the primary condition or disability, and the athletic trainer and other members of the sports medicine team may overlook medical issues that exist other than the primary disability. This is known as diagnostic overshadowing.5,6 Patel and Greydanus6 stress the importance of a detailed history and suggest that the PPE be completed by a sports medicine team that is involved in the athlete’s long-term care and that know the athlete’s baseline functioning. It is also suggested that the mass or station method of the PPE be avoided with these athletes. The decreased mobility of some athletes also makes this method impractical. Specific recommendations are that medical personnel talk at eye level with an athlete in a wheelchair, ask the athlete what movement patterns are possible and which parts of the body have normal sensation, and be cognizant of skin conditions and pressure sores.7 Athletes with histories of seizures are known to have 2.5 times the rate of injury compared with those with no seizure history.8 This further necessitates a careful PPE during which the clinician obtains information such as seizure history and documentation of antiseizure medication, including type, dose, use frequency, and side effects related to performance.
Many athletes with spinal cord disabilities have constant or residual pain from their disability and may not be able to distinguish pain due to a sport injury from pain due to a disability. Having a baseline appreciation for the athlete’s unique condition will help the assessment process should a general medical condition arise later. An additional component of the examination includes careful evaluation to ensure proper fit and adequacy of any prostheses, orthoses, sports wheelchairs, or other assistive devices being used by the athletes. Stump care in athletes with amputations, and bowel and bladder habits in athletes with spinal cord injury, will also be in the athletic trainer’s purview of questioning during the PPE. It may be necessary to have a parent or guardian present to assist obtaining an accurate history from an athlete with an intellectual disability (e.g., Special Olympics athlete). Athletic trainers seek assistance from professionals with expertise in the specific area of disability. Other aspects to consider during the PPE for an athlete with a disability are listed in Box 19-1.
To identify medical problems, provide baseline data, and to identify training goals, Jacob and Hutzler9 developed the Sports-Medical Assessment Protocol (SMAP), a tool for the evaluation of athletes with neurological disabilities. The SMAP includes a clinical interview, cardiopulmonary testing, and physical and functional assessments. Concerning participation guidelines, the American Academy of Orthopaedic Surgeons developed a sport participation possibility chart designed to provide initial guidance for the athlete and sports medicine team regarding which sports are appropriate. Factors influencing how to match an athlete to a particular sport include the psychological maturity of the athlete, adaptive and protective equipment, modification of the sport, the athlete’s and parents’ understanding of the inherent risks of injury, current health condition of the athlete, and level of competition and position played.6 The following questions could be asked of athletes with particular disabilities during the PPE5:
1. Does the athlete have a history of seizures, hearing loss, or vision loss? Are the seizures controlled? These are common abnormalities seen in Special Olympic athletes. Uncontrolled seizures often require a consultation with a neurologist and a delay in clearing the athlete for sports participation.
2. Does the athlete have a history of cardiopulmonary disease? Congenital cardiac disorders, including heart murmurs, ventricular septal defects, and endocardial cushion defects, are more common in persons with Down syndrome.
3. Does the athlete have a history of renal disease or unilateral kidney? Various renal anomalies, such as hypoplasia, dysplasia, and obstruction, are more common in people with Down syndrome.
4. Does the athlete have a history of atlantoaxial instability? Spontaneous or traumatic subluxation of the cervical spine is a potential risk in athletes with Down syndrome.
5. Has the athlete had heatstroke or heat exhaustion? Thermoregulation in athletes with spinal cord injuries is impaired because of skeletal muscular paralysis and a loss of autonomic nervous system control. Medications used for pain and bladder dysfunction can interfere with the normal sweat response.
6. Has the athlete had any fractures or dislocations? Ligamentous laxity and joint hypermobility are prominent features in athletes with Down syndrome.
7. What prosthetic devices or special equipment does the athlete use during sports participation? Health care providers need to be aware of an athlete’s need for adaptive equipment and regulations concerning their use in different sports.
8. Does the athlete use an indwelling urinary catheter or require intermittent catheterization of the bladder? Athletes with spinal cord injuries or other neurologic disorders often have bladder dysfunction or neurogenic bladders.
9. Does the athlete have a history of pressure sores or ulcers? Athletes who use wheelchairs are prone to pressure ulcers at the sacrum and ischial tuberosities, and athletes who use prostheses are prone to pressure ulcers at prosthesis sites.
10. At what levels of competition has the athlete previously participated?
11. What is the athlete’s level of independence for mobility and self-care?
12. What medications is the athlete taking?
13. Is the athlete on a special diet?
14. Does the athlete have a history of autonomic dysreflexia? This is an acute, potentially life-threatening syndrome of excessive, uncontrolled sympathetic output that can occur in athletes with spinal cord injuries at or above the sixth thoracic spinal cord level.
Overview of Anatomy and Physiology
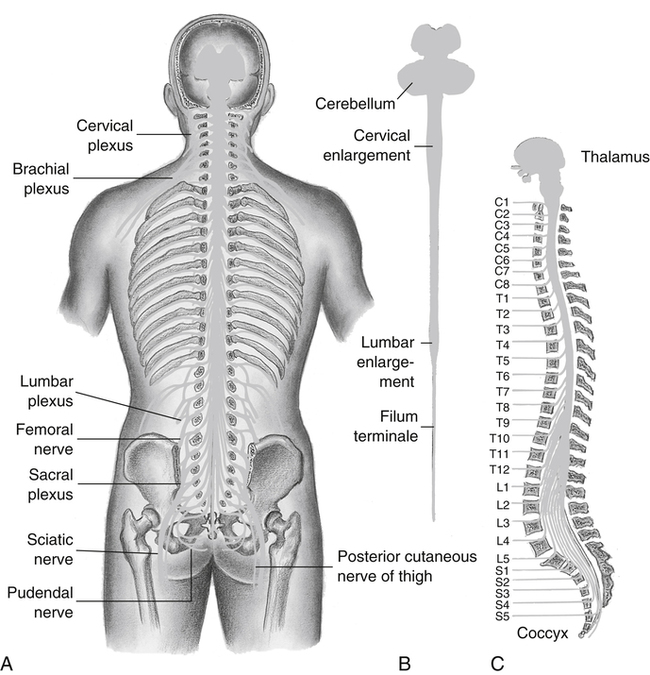
The anatomy and physiology pertinent to traumatic spinal cord injury are also pertinent to spina bifida, poliomyelitis, and cerebral palsy. The reader is referred to Chapters 11 through 13 and 17 to review the anatomy and physiology related to intellectual and sensory disabilities and amputation. Recall from Chapter 11 that the spinal cord is a cylindrical mass of nerve tissue that consists of 31 pairs of spinal nerves and extends from the medulla to the first or second lumbar vertebra. It is protected by the vertebral column, which consists of 7 cervical, 12 thoracic, 5 lumbar, 5 fused sacral, and 4 or more fused coccygeal vertebrae (Figure 19-1). The lumbar and sacral roots of the spinal cord fan out like a horse’s tail at L1-L2, giving rise to the term cauda equina.
Pathological Conditions
Spinal cord disability results from some form of injury or disease to the vertebrae and/or the nerves of the spinal column. Usually some degree of paralysis accompanies the disability. The degree of the paralysis is a function of the location of the injury on the spinal column (e.g., C5) and the number of neural fibers that are destroyed as a result of the injury. The level of function for an athlete with a spinal cord disability depends on the level of the lesion. The lower the level of the lesion on the spinal cord, the greater the functional ability of the athlete. Table 19-1 provides a summary of the relationship between level of lesion and functional ability. In addition, note that athletes with lesions above S2 will experience bowel and bladder concerns. It is appropriate for the clinician to discreetly ask the athlete how bowel and bladder issues are controlled.
TABLE 19-1
Level of Spinal Cord Lesion Related to Functional Ability
| Level of Lesion | Functional Abilities |
| C4 | Use of neck and diaphragm |
| Needs total assistance for transfers | |
| Limited respiratory endurance | |
| Controls electronic wheelchair by mouth-operated joystick | |
| C7 | Ability to extend elbow and flex and extend fingers |
| Uses wheelchair independently | |
| Transfers to some extent independently | |
| Weak grasp | |
| T1-T9 | Ability to use upper extremities |
| Little or no use of lower extremities | |
| Lower level injury: some control of upper back, abdominal, and rib muscles | |
| Lower level injury: may ambulate with braces | |
| T10-T12 | Complete control of upper back, abdominal, and rib muscles |
| Ambulates mainly with use of long leg braces or crutches | |
| Uses wheelchair for convenience; wheelchair sport possible | |
| L1-L3 | Hip joint flexibility and ability to flex hip |
| Ambulates independently with short leg braces, cane, or crutches | |
| S1 | Ability to flex knees and lift foot |
| Ambulates without crutches but may require ankle braces or orthopedic shoes |
Traumatic Tetraplegia and Paraplegia
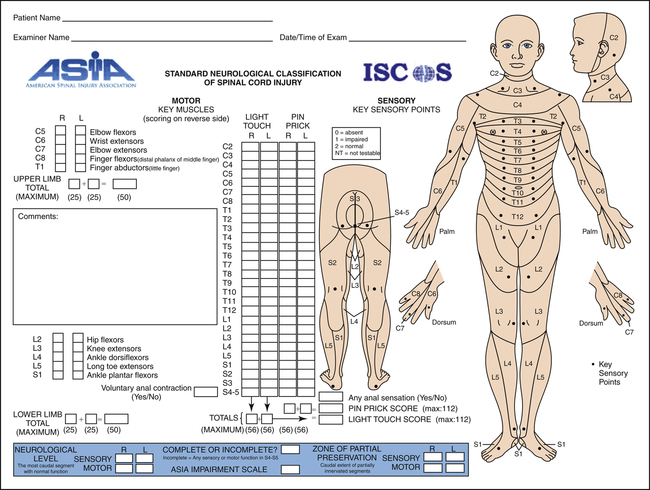
The extent of an SCI is generally described by means of a five-point grading system. The American Spinal Injury Association (ASIA) advocates an impairment scale according to which an athlete will be classified as ASIA A (complete), ASIA B (incomplete with sensation only), ASIA C (incomplete with nonfunctional motor ability), ASIA D (incomplete with motor function), and ASIA E (normal motor and sensory function). A full explanation of this impairment scale can be found in Figure 19-2. An athlete with complete tetraplegia (formerly called quadriplegia) has a lesion above T1 that involves the cervical spine and affects all four limbs, has no trunk control or sitting balance, and usually uses a high-backed sports wheelchair. The athlete will also be strapped in for safety. An athlete with complete paraplegia has a lesion below T1 that affects the lower extremities, has full use of the upper extremities, and may or may not have trunk control and sitting balance. Training apparatus is available to encourage correct movement.

Athletes with SCI have higher resting heart rates and lower blood pressures than their able-bodied counterparts. The higher heart rate is due primarily to the smaller venous return from the nonworking muscles in the lower extremities. To maintain cardiac output when stroke volume is less, the heart compensates by increasing its rate. In turn, the blood pressure lowers to accommodate a decreased perfusion need in the nonworking limbs. During the general medical assessment, the clinician is aware that the baseline blood pressure for adults with tetraplegia may be as low as 90/60 mm Hg and that peak heart rates for persons with tetraplegia typically do not exceed 130 beats per minute. As part of the PPE, blood pressure should be obtained in two of three positions: supine and sitting or standing, as applicable.10 A change of less than 10 mm Hg between positions is acceptable. Resting pulse is checked, as tachycardia (i.e., heart rate >120 beats/min) may be the only sign of pneumonia or pulmonary embolus in an athlete with a high-level SCI.
Autonomic Dysreflexia
Autonomic dysreflexia (AD), also known as hyperreflexia, is a clinical phenomenon unique to individuals with SCI above the major sympathetic nervous system outflow tract. It is a potentially life-threatening complication from SCI particular to those with lesions above T6, although attacks are possible in athletes with lesions to T10.11 With AD, the athlete’s blood pressure rises to dangerous levels and, if not treated, can lead to stroke or death.
AD is an imbalanced reflex sympathetic discharge that occurs when there is a painful, irritating, or even strong stimulus below the level of injury, such as an insect bite, bone fracture, or distended bladder (Box 19-2). Intact peripheral sensory nerves transmit impulses that stimulate sympathetic neurons in the spinal cord below the level of the lesion. The inhibitory outflow above the SCI is increased but it is unable to pass below the block of the SCI. The large sympathetic outflow causes release of various neurotransmitters such as norepinephrine and dopamine, causing a sudden rise in blood pressure. Vasomotor brainstem reflexes attempt to lower blood pressure by increasing parasympathetic stimulation to the heart through stimulation of the vagus nerve, resulting in bradycardia. Because the parasympathetic system’s messages cannot pass through the SCI, the blood pressure remains elevated. The most common cause of AD is impairment in the urinary system. Everyday training and rehabilitation sessions have the potential for provoking AD.
Signs and Symptoms
possible for signs or symptoms to be absent despite the elevated blood pressure. It is paramount that the health care provider take a blood pressure reading when AD is suspected. Special attention is paid to any blood pressure more than 20 mm Hg to 40 mm Hg above the reference range for systole. The athletic trainer must know the athlete’s normal blood pressure so that changes can be detected. The heart rate should also be monitored for atypical decreases. An athlete may present with flushed skin, particularly in the face and neck area, and piloerection. The athlete may also have a headache or nasal congestion in response to the high blood pressure. Because impacted bowels and distended bladders can lead to AD, the clinician should also inquire about bowel and bladder habits.
Prevention
Prevention is the key with AD, and because most episodes result from forgetfulness or carelessness about urine needs,12 athletes should inspect catheters and void the bladder before activity. Athletes must be asked what type of bowel and bladder management program they maintain. They should avoid the risk of sunburn by using sunscreen, wearing hats or visors, seeking shade during nonactivity, and covering susceptible areas. Careful inspection after training or competition by the athlete and the athletic trainer for contributors such as blisters, sunburn, pressure sores, and constricting clothing or equipment will aid in preventing episodes of AD. To date, no specific relationship has been documented between AD and age. Because more males sustain SCI, AD is primarily a male phenomenon.
Boosting
Boosting is a dangerous technique used by some athletes with SCI in an attempt to gain an advantage over an opponent or to improve race times. Although this condition is potentially life-threatening, many athletes have intentionally induced AD in order to gain better performance results from increased blood circulation. Athletes with SCI can induce AD by holding their urine or clamping their catheters before an event, sitting on a sharp object (e.g., tack), strapping their legs very tightly, or aggressively pinching or striking themselves. Self-induced lower leg fractures have also been reported.6
Improvements of approximately 10% in track and swimming times have been reported in athletes with disabilities who used this tactic.13,14 This would be equivalent to reducing the able-bodied 26-mile marathon record by 12 minutes! However, research has reported this practice is, at the very least, unethical and must be prevented at all costs.15 The International Paralympic Committee bans boosting as a method of doping. The athletic trainer must know that this is an extremely risky behavior and must strongly discourage it. Athletes may think they are only receiving a catecholamine response and heart rate reserve that could normally be attained if uninjured. However, the significant rise in blood pressure that occurs with AD cannot be controlled by the body’s autonomic nervous system. Other factors, such as inadequate hydration, fatigue, thermal stress, illness, or anxiety, can add to the AD and push the athlete to the point of autonomic shutdown, which can lead to death.15 Athletes who have boosted will typically present at the starting line with profuse sweating, goose flesh, and significant leg spasms. These athletes should be immediately suspected. The athlete’s blood pressure is monitored and those with elevated measures are given 10 minutes to bring it down; if a diagnosis of boosting is confirmed, the examiner may withdraw the competitor from the event. Athletes anecdotally report that boosting is commonplace and is even obvious when watching competitors at the starting line.11
Thermoregulation Concerns
Evaporative cooling is the most effective heat loss mechanism for the body, providing more than 80% of heat loss in the able-bodied athlete. Individuals with spinal cord injury are unable to depend on the autonomic nervous system to lower their core temperature by regulating blood flow. In addition, sweating is often impaired below the level of the spinal cord lesion, requiring the athlete’s body to rely on less surface area for evaporative cooling.10 Therefore athletes with SCI are at greater risk of heat illness than their able-bodied counterparts, and the athletic trainer needs to keep careful watch over athletes during high-energy sports that raise their core temperature (Figure 19-3). Individuals with tetraplegia and those with lesions above T6 are particularly vulnerable to heat illness because they are unable to increase heart rate to sustain cardiac output when blood must flow to both the muscle and the skin.7,16
Likewise, in cold conditions these athletes may lack normal warming mechanisms, such as producing piloerection, shivering, and circulatory shunting. A lack of working muscle mass below the level of lesion contributes to temperature regulation problems. Even temperatures around 50° F (10° C) may pose problems for an athlete with a cervical or high thoracic lesion.17 Impaired or absent sensation intensifies the risk of hypothermia because these athletes may be unaware that damp clothing increases the loss of body heat (Box 19-3).
Signs and Symptoms
irritability, fatigue, headache, weakness, dizziness, decreased performance, erratic wheelchair propulsion, flushed skin, head or neck heat sensations, vomiting or nausea, and general discomfort. In able-bodied athletes chills and muscle cramps are common; such signs may not be present in athletes with SCI if piloerection is impaired. Usually muscle cramps are common in the gastrocnemius and abdominal muscles, but these muscles are often nonworking in individuals with SCI.
Heat stroke, a life-threatening emergency, is characterized in the able-bodied population as having a core temperature of 104° F (40° C) or higher18; a rapid increase in pulse is present as well (160-180 beats/min). The chief symptom of heat stroke is central nervous system dysfunction. Other symptoms are similar to those associated with concussions, namely confusion, agitation, inappropriate behavior or language, apathy, vacillating emotions, stupor, and coma or death if untreated.
Referral, Diagnostic Tests, and Differential Diagnosis
Thermoregulatory problems are often incorrectly attributed to athlete fatigue, illness, hypoglycemic reactions, concussion, or head injury.18 To determine hyperthermia, the clinician feels for hot skin of a distressed athlete. Because the individual with SCI has a decreased ability to regulate blood flow beneath the lesion, rectal temperatures may not be as accurate for measuring core temperature in athletes with spinal cord injuries.19,20 In addition, when the thermoregulatory system is impaired, typical signs such as shivering might not be observable. It is critical that the health care provider be able to review a thorough medication and nutritional supplement history for the athlete that includes prescription and over-the-counter products. Sympathomimetics and anticholinergics affect thermoregulation, as do diuretics and excessive caffeine.6 Emergency referral is warranted if the athlete is not responding to treatment or if heat stroke is suspected.
Treatment
Treating thermoregulatory problems in the athlete with a spinal cord disability is similar to treatment for the able-bodied athlete. For hyperthermia, the athlete is moved to a shaded or cooled area, clothing is loosened, equipment removed, oral fluids are administered, and cooling is accomplished with cold water. Intravenous fluids are administered if the athlete is not coherent. If heat stroke is suspected, emergency measures to reduce the athlete’s temperature (e.g., emersion in cold water bath or sponge cool water, fanning body with a towel) are performed first; then the athlete must be transported to an advanced emergency care facility.20 It should be stressed that although immersion in an ice bath has been recommended for able-bodied athletes, this treatment should be used with caution in the athlete with a spinal cord injury, especially one with a complete, high level of lesion because the thermoregulatory system is impaired. Cooling may occur too rapidly. To date, research in this area for athletes with spinal cord injury is incomplete. Therefore, indications and contraindications should be discussed with the physician who is most familiar with the individual athlete.
Prognosis and Return to Participation
The National Athletic Trainers’ Association established an Inter-Association Task Force that created a statement on exertional heat illness containing return-to-activity guidelines for able-bodied athletes.20,21 In general, these guidelines include clearance by a physician and a gradual and monitored return to activity, and may be used for the athlete with SCI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree