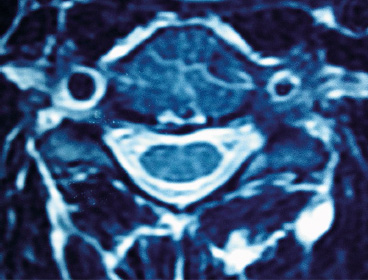
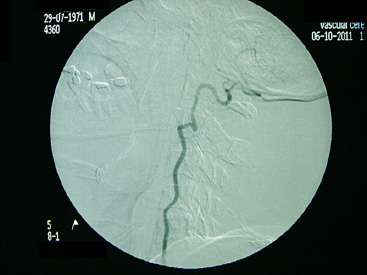
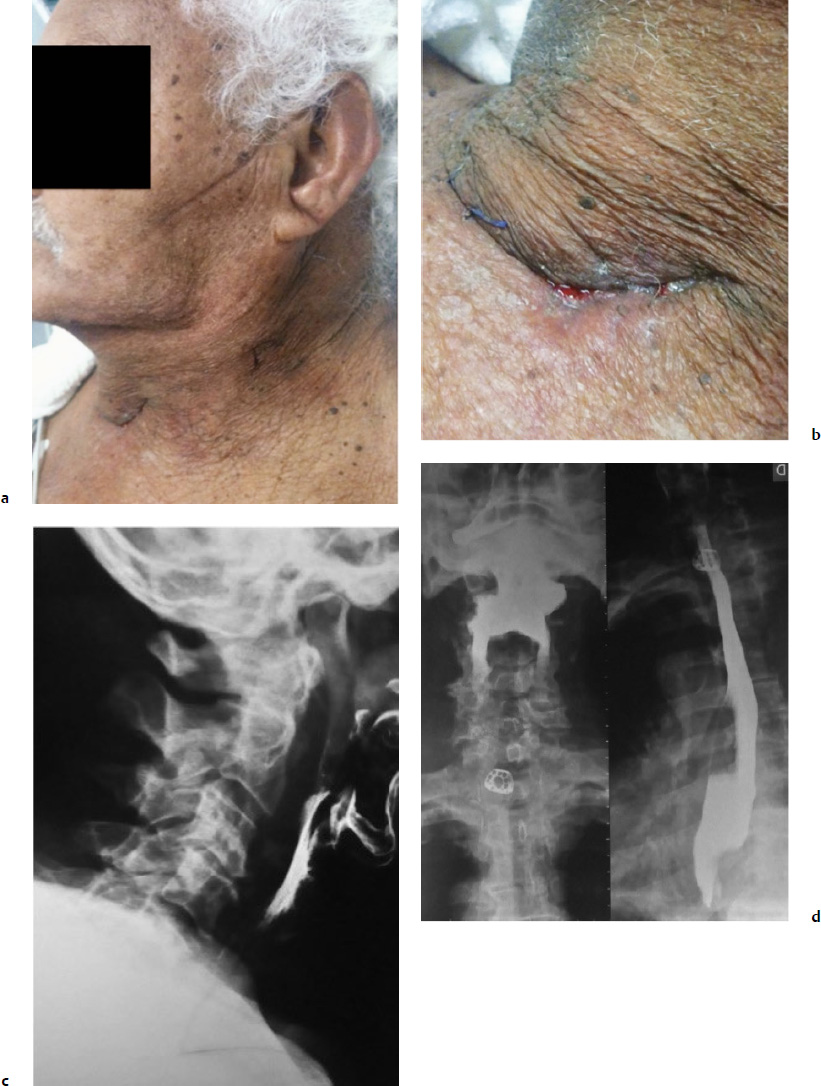
9 Anterior cervical spine surgery currently represents one of the most common procedures performed by spine surgeons.1 Reported data from North American National Hospital Discharge Survey (NHDS) database, between 1990 and 2004, identified more than 770,000 cases of anterior cervical diskectomy and fusion (ACDF)—an increase of eightfold in the number of procedures (up to 28-fold when considering only individuals older than 65 years).2 Similarly, the National Inpatient Sample (NIS) database reports more than 1,100,000 cervical surgeries in the North American population between 1998 and 2006, 91.7% of which were anterior procedures, mostly ACDF.3 Given the importance of ACDF in the current clinical practice of spinal surgeons, it must be kept in mind that anterior approaches to the cervical spine are not innocuous procedures. In a literature review, Fountas et al4 analyzed the outcomes of 1015 cases of ACDF and reported a complication rate of 19.3% (196 of 1015 patients). The most common complication in this series was dysphagia, present in 9.5% of cases. Wang et al5 retrospectively analyzed the complication incidence in 932,000 cervical surgeries, of which 73% were ADCF, reporting a complication rate of 3.1%, and emphasized some distinct patterns in the pathophysiology of adverse events: (1) systemic complications related to preexisting comorbidities or identifiable risk factors, which could have been worsened or triggered by surgical/anesthetic procedures; (2) local complications related to the surgical technique, implant, or specific pathology (e.g., complications directly related to disk arthroplasty, or to specific pathologies such as diffuse idiopathic skeletal hyperostosis [DISH], ossification of the posterior longitudinal ligament [OPLL]); (3) local complications related specifically to the anterior approach to the cervical spine (e.g., dysphagia, dysphonia, and vertebral artery or esophageal lesions).5 This chapter discusses the most common surgical situations from a practical point of view, focusing on the pathophysiology of the lesions, identifying the avoidable risk factors, and formulating management strategies to minimize complications. Iatrogenic injury to the vertebral arteries (VAs) represents an uncommon event when performing surgery on the cervical spine from the anterior approach, but it is a complication that is difficult to manage and it may lead to serious consequences. The reported incidence varies from 0.3% to 0.5%, significantly less than its incidence in cervical spine surgery from the posterior approach, in which the incidence is reported to be up to 8%.6 The VA usually originates from the subclavian or innominate arteries, running in an anterolateral direction in relation to the midline, until it enters the transverse foramen at the level of the sixth vertebra. At the base of the axis, the VA turns in a posterolateral direction, passes through the C1 transverse foramen, perforates the posterior atlantoaxial membrane, and enters the foramen magnum to join it as well as the originating basilar artery.7 In the anterior approach, the VA is especially vulnerable to injury at two points: (1) in the space between its origin from the subclavian artery and its entrance into the transverse foramen, in which it gets exposed in an anterolateral position; and (2) after its entrance into transverse foramen, at the intertransversal spaces.8 The following factors have a strong correlation with an increased risk of injury: (1) aggressive bone or disk resection, especially using a drill in a far-lateral position from the midline; (2) pathological alteration of bone structure or quality, often due to infection or tumor; and (3) VA abnormalities.1,6,8 There are some anatomic landmarks of particular importance in determining the safe work space in anterior procedures. Marking the limits of medial insertions of the longus colli in the vertebral bodies before dissection or placing retractors helps to orient the surgeon to the midline. To define the lateral working limits, the uncovertebral joints can be used as a landmark, enabling a safe dissection up to the medial border of the uncinate process (UP), if no VA abnormalities were noted preoperatively. The medial border of the transverse process is located at a mean distance of 5 to 6 mm laterally to the uncinate process.8 Kim et al9 evaluated the anatomic relationship between the uncinate process and the adjacent structures, verifying a mean distance of 4.2 to 5.7 mm from the most anteromedial point of the UP to the medial border of the transverse foramen, which the authors found to be a safe landmark of the lateral limit. In procedures that require a more aggressive approach to the foraminal space, direct visualization and the use of the proper instruments are essential, as is recognizing the decreased safety margins, ranging from 1.7 to 2.4 mm from the lateral border of the UP to the medial border of the transverse foramen. The presence of anatomic variants or abnormalities can substantially increase the risk of injury. The rate of VA anatomic abnormalities is reported to be 10% in larger series.10 Two VA abnormalities are associated with an increased risk of intraoperative injury: (1) medial migration of the VA, especially due to intrinsic tortuosity of the vessel; and (2) an atypical entry of the VA into the transverse foramen, often at levels cephalad to C6, thus being exposed along a major segment in an anterolateral position to the vertebral body.7 Anatomic studies analyzing the patterns of VA abnormalities found that VA medial migration (defined as a distance from the uncovertebral joint of less than 1.5 mm) occurred in 11.6% of cases, of which 4.4% occurred at the C3 level and a larger medial deviation of 5.6 mm was also found.7 Furthermore, a significant correlation was found between increased age and the presence of VA medial migration, thus supporting the hypothesis of a cervical spondylosis occurring with the loss of normal intersomatic relationships, and thus subjecting the patient also to the occurrence of tortuosity. An abnormal entry of the VA into the transverse foramen was found in 8% of patients, occurring cephalad to the usual level (C6); only 26% of the arteries evaluated had the same diameter, and in almost 10% of cases a unilateral hypoplasia (arterial lumen less than 2 mm) was found.7 Regarding the indications for preoperative imaging for angiographic evaluation, Sano et al11 performed tridimensional computed tomography angiography (CTA) to evaluate the VA structure, including its collateral circulation to the basilar artery. Eleven percent of patients exhibited hypoplastic arteries. The absence of competent arterial collateral vascularization was seen in 47% of cases. Another 7% of patients were found to have an abnormality that the authors referred to as a “critical” VA, that is, a single and hypoplastic artery with the absence of functional collateral vascularization (Fig. 9.1). Fig. 9.1 Preoperative magnetic resonance imaging (MRI) showing a left vertebral artery (VA) markedly hypoplastic and a wider right VA closer than usual to the stenotic foramen that is planned to be approached. Fig. 9.2 Preoperative angiography showing suspected abnormal VA with tortuosity and medial migration of the vessel, protruding into the disk space. The use of advanced imaging techniques is not always readily available and is associated with a significant increase in radiation exposure and costs. The authors recommend using CTA or magnetic resonance angiography (MRA) in cases with factors known to increase the risk of VA injury: • Structural bone abnormality • Upper cervical pathology • Traumatic instability • Tumor mass involving the VAs Once these abnormalities are detected in preoperative evaluation, preventive measures still can be taken, delaying preoperative planning until the definitive treatment of the vascular pathology before or even during the same surgical procedure, if possible (Fig. 9.2). Routine magnetic resonance imaging (MRI) can yield some important information about VA status: • Presence of a unilateral or hypoplastic artery • Presence of tortuosities or medial migration of the VA • Abnormal entry of the VA into the intertransversal foramen • Tissue abnormalities caused by infection or a tumor that alters bone consistency as well as the anatomic landmarks. • In cases of a detected or highly suspicious abnormality, we evaluate the abnormality with angiography or CTA, which helps determine precise diagnosis and thus prepare alternative plans. • Limit the lateral dissection in areas of more exposure of the VAs (below C6 or higher in cases of abnormal entry in the intertransverse foramen). • Diskectomy and corpectomy: limit the lateral boundaries to the medial border of the uncinate process; if foramen exploration is required, it must be done with the appropriate instruments under direct visualization. The lateral approach, as with a foraminotomy, should be considered. • We strongly recommend not to use a drill beyond the medial borders of the UP. • Avoid placement of sharp retractors (Homan’s retractors) in the intertransverse spaces. Regardless of the method of treatment, the fundamental points to consider are localization and appropriate exposure of the injury. The goals of primary management are immediate bleeding control, prevention of acute vertebrobasilar ischemia, and prevention of cerebral ischemic phenomena. Also, maintenance of cerebral perfusion pressure is crucial, which in some cases requires aggressive fluid and blood transfusion. Most reported cases late hemorrhage due to VA injury presented with the development of a fistula or pseudoaneurysm.12 Two situations are identified at the initial event: the injury was not recognized intraoperatively, or an injury was insufficiently or ineffectively treated.13 Tamponading the source of bleeding with sponges or hemostatic agents is the first step in controlling it, and extensive blood loss should be expected, which engenders higher complication rates in the early postoperative period. Intraoperative angiography helps determine the diagnosis of the injury, and its localization and extension. Intraoperative angiography also evaluates the contralateral VA, thus helping to establish the initial action plan. In situations where the surgeon plans to perform a definitive occlusion, it is recommended that an intra-operative angiography be done to confirm the patency of the contralateral artery. In cases of a repairable injury, the bleeding must be controlled appropriately, which might require the services of a vascular surgeon. Theoretically, direct repair is the best method of management of a VA injury; however, it is a technically demanding procedure, and in many cases it is not feasible to preform it. We recommend first that a direct repair be performed whenever possible; if it is not possible, we then recommend performing a definitive occlusion with the goal of preventing late complications, especially fistulas and pseudoaneurysms.12,13 Ligation can be done in cases of extensive injuries in which repair is not feasible (extensive lacerations by drilling, complete section with vessel retraction, severe atherosclerotic disease) or in cases in which it is difficult to control bleeding in a documented patent contralateral VA. Although rare, an esophageal injury may have serious consequences and result in a life-threatening condition. The injury might be diagnosed as late as 10 years after the index surgery.14 The injuries can be categorized as intraoperatively iatrogenic; occurring late due to implant failure and migration; and traumatic, with a late presentation.15 The reported incidence of these injuries varies between 0.2% and 0.4% in larger series. There is a significant association with anterior instrumentation, especially in multilevel surgery, corpectomy, and trauma.1,15 The main cause of esophageal injury is anterior cervical instrumentation. Technical issues should also be considered in determining the pathogenesis, such as aggressive dissection with sharp instruments, the use of static retractors with excessive pressure, an inadvertent surgical injury, and traumatic orotracheal intubation. Furthermore, patients with a previous history of cervical trauma, digestive tract pathology, or structural compromise may have an increased risk of injury.16 The clinical presentation of an esophageal injury varies, based on the length of time between the occurrence and the diagnosis. The therapeutic strategy depends on the presentation. Acute injuries can be recognized intraoperatively and promptly managed. But other patients present with severe deterioration of the clinical status in the early postoperative period, up to 48 hours. Late injuries may occur long after the index surgery; these cases are generally related to the failure and migration of the anterior cervical instrumentation.15,16 Diagnosis demands a high degree of suspicion, especially in cases of acute injury that can be seen intraoperatively. When the surgeon is not able to locate the injury, an intraesophageal dye or contrast injection with fluoroscopic visualization might be helpful. If an intraoperative injury is not recognized and managed immediately, patients may exhibit clinical deterioration in the early postoperative period. The clinical features often include dysphagia, odynophagia, subcutaneous emphysema, prevertebral soft tissue swelling, fever, and dyspnea. Some cases may present with digestive tract secretion drainage, including the presence of alimentary residual fragments extruding through the operative wound. The most severe cases may present with mediastinitis, sepsis, and even death. In cases treated in the first 24 hours, the mortality rates can range up to 20%, and even to 50% when treatment is delayed beyond the first 24 hours.16 In cases of late injury, there is often failure or migration of anterior implants. The clinical presentation may be more variable, ranging from severe symptoms of sudden onset, similar to the presentation of an acute injury, to chronic cases presenting with recurrent episodes of dysphagia, fever, and pneumonia.16 A variety of complementary exams, including laboratory and imaging, can be of great utility in the diagnosis and treatment planning. Contrast esophagram and barium swallow studies enable the detection and localization of injuries or fistulas. An upper digestive tract endoscopy can give more detailed information about lesions and tissue conditions. Imaging studies, including conventional radiographs, are fundamental for the evaluation of implant conditions, especially in late injury (Fig. 9.3). • Surgical planning must consider the feasibility of the anterior approach in cases of local pathology or adverse anatomy, such as revision surgery, previous neck surgery, hyperthyroidism, tumor, obesity, and diverticular or metaplastic esophageal disease. • Placement of a nasogastric catheter facilitates localization of the esophageal wall and thus appropriate protection during the procedure. • The use of manual retractors instead of autostatic instruments, achieving proper placement under the longus colli muscle belly, and avoiding fragile areas of the esophageal wall improve the surgical outcome. • Attention to the technical issues, such as careful blunt dissection without the use of sharp instruments, proper placement of anterior implants and avoiding their protrusion through the esophageal wall, and avoiding excessive use of electrocautery in deep dissection improve the surgical outcome. Once an injury has been diagnosed, treatment must be initiated immediately. In injuries recognized intraoperatively, direct repair is the best choice whenever possible. There are some variation in the technical issues that can be encountered, such as the use of one- or two-layer sutures, the use of circumferential reinforcement sutures, and the indications for and results of the use of muscle flaps, especially the longus colli, sternocleidomastoid, and pectoralis major.17,18 Several clinical measures are of crucial importance and must be promptly instituted in all cases: (1) nasoenteral feeding for at least 7 to 10 days; (2) catheter removal and gradual return to oral feeding after documenting the absence of any extravasation on a contrast esophagram; (3) broad-spectrum antibiotic therapy, in which the duration of treatment and the need for antibiogram orientation must be determined by a multidisciplinary term including a specialist in infections.16–18
Complications of Anterior Surgery: Vertebral Artery Injury, Esophageal Perforation, and Dysphagia
 Introduction
Introduction
 Vertebral Artery Injury
Vertebral Artery Injury
Anatomic Variations and Abnormalities
Management Strategies to Prevent Complications
Preoperative Planning
Imaging
Intraoperative
Maintenance of a Safe Working Space
Management of an Intraoperative Lesion
Controlling Bleeding
Performing Intraoperative Angiography
Direct Repair
Ligation
 Esophageal Injury
Esophageal Injury
Risk Factors
Clinical Presentation and Diagnosis
Management Strategies to Prevent Complications
Preoperative Planning and Intraoperative Measures
Management of Intraoperative and Late Injuries
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree