109 Bacterial Arthritis
Most instances of native joint infection are the result of bacteremic seeding.
Staphylococcus aureus is the most frequent microorganism in adult nongonococcal septic arthritis.
Epidemiology
Bacterial infections of the joint are usually curable with treatment, but morbidity and mortality are still significant in patients with underlying rheumatoid arthritis (RA), patients with prosthetic joints, elderly patients, and patients who have severe and multiple comorbidities. Goldenberg1 wrote in 1994, “Treatment and outcome [of septic arthritis] have not improved substantially over the past 20 years.” This statement is probably still true today. Incremental knowledge of the pathogenesis of septic arthritis caused by two common organisms, Neisseria gonorrhoeae and Staphylococcus aureus, and understanding of the pathobiology of prosthetic devices may lead to innovations in the management and prevention of bacterial joint infections.
The normal diarthrodial joint is resistant to bacterial infection because of local and systemic host defenses. Bacteria can reach the synovial-lined joint, however, via the hematogenous route and result in septic arthritis. The large joints are affected more commonly than the small joints, and monoarticular infection is the rule, with polyarticular infection (more than one joint involved) in less than 20% of cases. A prospective series from a community-based population in the Netherlands reflected a representative distribution of joint involvement: knee 55%, ankle 10%, wrist 9%, shoulder 7%, hip 5%, elbow 5%, sternoclavicular joint 5%, sacroiliac joint 2%, and foot joint 2%.2
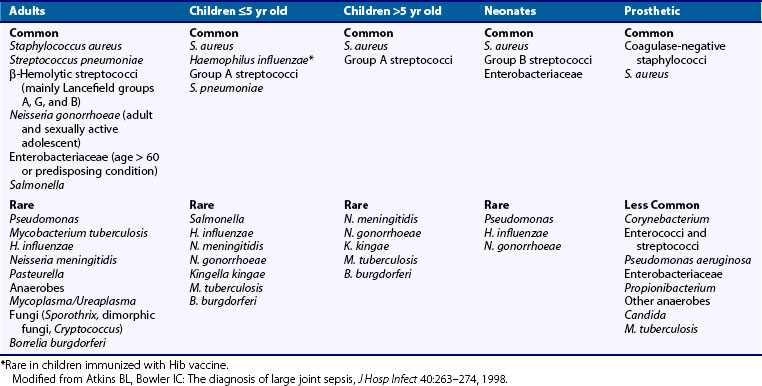
The incidence of septic arthritis ranges from 2 to 5/100,000/year in the general population, 5.5 to 12/100,000/year in children, 28 to 38/100,000/year in patients with RA, and 40 to 68/100,000/year in patients with joint prostheses.3,4 The incidence appears to be increasing, probably related to orthopedic procedures, an aging population, and the increased use of immunosuppressive therapy.5 The organisms causing bacterial arthritis depend on the epidemiologic circumstances (Table 109-1). Monoarthritis of a prosthetic joint in an elderly man is likely due to Staphylococcus, whereas a migratory arthritis in a sexually active woman with skin lesions is likely due to disseminated gonococcal infection. Septic arthritis caused by methicillin-resistant S. aureus (MRSA) is common in the elderly, in persons who use intravenous drugs, and in individuals with prosthetic joints.6
Etiology
Table 109-1 lists the common organisms that cause joint infections according to the age of the patient and whether the joint is native or prosthetic.5 Overall, S. aureus is the most common etiologic agent among children of all age groups, followed by group A streptococci and Streptococcus pneumoniae.7 Neonates and infants younger than 2 months old are more susceptible to group B streptococci and gram-negative enteric bacilli than older children. Rarely, Pseudomonas, N. gonorrhoeae, and Candida albicans may be responsible in very young children. Since the introduction of the Haemophilus influenzae type B vaccine, the incidence of septic arthritis caused by H. influenzae has declined dramatically.8 In sexually active adolescents, N. gonorrhoeae must be considered. Pseudomonas aeruginosa and Candida are potential pathogens in adolescent intravenous drug abusers. Patients with sickle cell anemia are prone to develop Salmonella arthritis, and immunocompromised children are at higher risk for infection with gram-negative bacilli. Other unusual joint pathogens in children include Neisseria meningitidis, anaerobes, Brucella, and Kingella kingae.
The organisms causing nongonococcal septic arthritis in adults are 75% to 80% gram-positive cocci and 15% to 20% gram-negative bacilli.9 S. aureus is the most common organism in native and prosthetic joint infections. Staphylococcus epidermidis is common in prosthetic joint infections but is a rare cause of native joint infections. The streptococci including Streptococcus pneumoniae are the next most common group of gram-positive aerobes. Streptococcus pyogenes is followed by groups B, G, C, and F in frequency. Patients with non–group A streptococcal disease often have comorbidities such as immunosuppression, diabetes mellitus, malignancy, and severe genitourinary or gastrointestinal infections.10 Group B streptococcal arthritis in adults is uncommon, but it can be a serious infection in adult diabetics and patients with late prosthetic hip infections.11 Aggressive polyarthritis caused by group B streptococci may result in serious functional damage and permanent morbidity.12 Patients predisposed to gram-negative bacillary infections include patients with a history of intravenous drug abuse, very young and very old patients, and immunocompromised patients.13 The most common gram-negative organisms are Escherichia coli and P. aeruginosa.
Anaerobes account for 5% to 7% of septic arthritis.2,3,14 Common anaerobes include Bacteroides, Propionibacterium acnes, and various anaerobic gram-positive cocci. Predisposing factors include wound infections, joint arthroplasty, and immunocompromised hosts. Foul-smelling synovial fluid or air in the joint space should raise the suspicion of anaerobic infection, and appropriate cultures should be obtained and held for at least 2 weeks. Anaerobes and coagulase-negative staphylococci are more common in prosthetic joint infections.
Polyarticular septic arthritis is much less common than monoarticular infection.15 Many of the patients have one or more comorbidities, and some have been intravenous drug abusers. Occurrence of polyarticular septic arthritis is high in patients with RA and averages 25% (range, 18% to 35%).16 Although S. aureus is the most common pathogen, group G streptococci, H. influenzae, S. pneumoniae, or mixed aerobic and anaerobic bacteria have been responsible for polyarticular infections. Involvement of more than one joint also can occur in certain patient populations such as neonates and patients with sickle cell anemia, or with certain organisms, such as N. gonorrhoeae, N. meningitidis, and Salmonella.17
Polymicrobial (two or more bacterial species), polyarticular (two or more joints) septic arthritis is a rare clinical entity.18 Large joints are usually affected. Among five reported cases, the knee was affected in four cases (bilaterally in two); the elbow and wrist were affected in three cases, and the shoulder was affected in two cases. The mean number of joints infected was three. Bacteremia was present in all but one case (80%) and always involved the same organisms that were in the synovial fluids. Most bacterial species isolated were the usual organisms seen in septic arthritis. Combinations of gram-positive aerobic and anaerobic organisms were common. A characteristic of most cases (80%) was the extension of locally destructive processes as a result of the contiguous spread of infection from the affected joints such as osteomyelitis, fasciitis with compartment syndrome, and abscess or sinus tract formation. Systemic complications including septic shock, multiorgan failure, and toxic shock syndrome were noted in 60% of cases. The mortality rate of polymicrobial, polyarticular septic arthritis in this small series was 60%.18
Arthrocentesis is a common procedure frequently used in conjunction with corticosteroid administration in patients with various forms of joint diseases. Septic arthritis after joint aspiration and injection is extremely rare, occurring in 0.0002% of patients.19 Arthroscopic surgery is also a common procedure that is complicated by a low incidence of septic arthritis (<0.5% of procedures).20 Coagulase-positive and coagulase-negative staphylococci account for more than 87% of these infections. In rare cases of septic arthritis of the knee related to anterior cruciate ligament repair, the tissue allografts were identified as the source of the infection.21 Cultures yielded gram-negative organisms such as Pseudomonas aeruginosa, Citrobacter, Klebsiella oxytoca, and mixed infection with S. aureus, Enterococcus faecalis, and P. aeruginosa.
Pathogenesis
Acute bacterial arthritis is usually designated gonococcal or nongonococcal. In the case of gonococcal arthritis, N. gonorrhoeae possesses a variety of virulence factors on the cell surface. N. gonorrhoeae is able to attach to cell surfaces via filamentous outer-membrane appendages, or pili. Another outer membrane protein, protein I, has forms IA and IB. Protein IA binds the host factor H and inactivates complement component, C3b, circumventing the host’s complement system.22 Protein IA also prevents phagolysosomal fusion in neutrophils, enabling survival of the organism within the phagocytes. Lipo-oligosaccharide is a gonococcal molecule similar to lipopolysaccharide of other gram-negative bacteria and possesses endotoxin activity, which contributes to the joint damage seen in gonococcal arthritis.23
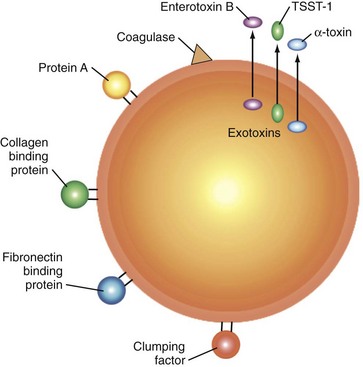
S. aureus is the most common organism that causes nongonococcal arthritis. The virulence of S. aureus is associated with its ability to attach to host tissue within the joint, evade host defenses, and cause damage to the joint. Table 109-2 lists some of these virulence factors and their mechanisms of action. The attachment of S. aureus to the joint tissues is facilitated by microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). MSCRAMMs are embedded in the cell wall peptidoglycan of S. aureus (Figure 109-1).24 They bind to host matrix proteins including collagen, fibrinogen, elastin, vitronectin, laminin, and fibronectin. Gene knockout experiments in animal models showed that the gene coding for the protein that binds collagen is an important virulence factor for S. aureus joint infections.25 Most S. aureus isolates also express the fibronectin-binding proteins, FnbpA and FnbpB. Disruption of the respective genes, fnbpA and fnbpB, by knockout gene experiments completely obliterates adherence of S. aureus to fibronectin-coated surfaces (e.g., prosthetic joints).26
Table 109-2 Virulence Factors of Staphylococcus aureus and Their Mechanisms of Action
| Virulence Factor | Mechanism of Action |
|---|---|
| Collagen-binding protein | Binds collagen |
| Clumping factor A and B | Binds fibrinogen |
| Fibronectin-binding protein A and B | Binds fibronectin |
| Capsular polysaccharide | Antiphagocytic |
| Protein A | Binds fragment crystallizable portion of IgG |
| Toxic shock syndrome toxin-1 | Superantigen |
| Enterotoxins | Superantigens |
The genes of several S. aureus cell surface proteins (e.g., protein A, fibronectin-binding proteins, coagulase) and exotoxins (e.g., toxic shock syndrome toxin-1 [TSST-1], enterotoxin B, proteases, and hemolysins) are regulated by the accessory gene regulator agr.27 At low cell numbers such as at the time of infection, production of cell surface proteins for attachment to host tissues is facilitated by the agr gene. When the cells have attached to tissue or an orthopedic device and have passed from exponential to stationary phase of growth, agr represses the expression of genes coding for cell surface proteins and activates genes coding for exotoxins and tissue-destroying exoenzymes. Because of this complex effect on the different stages of infection, inhibitors of agr may reduce tissue destruction but enhance tissue colonization. This effect could have implications for chronic infections such as occur with prosthetic joints.
Adherence receptors may allow intracellular movement of S. aureus into host cells (e.g., osteoblasts, endothelial cells, neutrophils).28 When internalized, the organism is protected from the host’s immune system and from antimicrobial agents. After adherence to the joint tissue, the bacteria activate the host immune response. Opsonization and phagocytosis are key defenses to eradicate the organism. S. aureus possesses two virulence factors, protein A and capsular polysaccharide, which interfere with these defenses. Protein A interferes with binding of complement by binding to the fragment crystallizable (Fc) portion of IgG. Protein A has been termed a superantigen for B cells because 30% of human B cells show Fab-mediated binding of the protein A molecule.29 Binding of protein A by B cells leads to activation and subsequently to depletion of B cells through apoptosis.30 This process may have implications regarding the ability of the immune system to control infection with S. aureus. The gene coding for protein A had been experimentally disrupted, and joint infection caused by the altered strain in a mouse model resulted in less joint destruction than infection caused by the wild-type strain.31
Capsular polysaccharide interferes with opsonization and phagocytosis. Of the 11 reported capsule serotypes of S. aureus, types 5 and 8 account for 85% of clinical infections.32 The capsule of these two serotypes is thinner, which facilitates the attachment to host fibronectin and fibrin.33 When attached to these host proteins, capsule production is upregulated to form a thicker capsule, which makes the bacteria more resistant to opsonization and phagocytosis. The thicker capsule can also conceal the highly immunogenic adherence proteins (MSCRAMMs).34 A mutant of the type 5 capsule in a murine model had a lower rate of infection and resulted in less severe arthritis compared with mice infected with the wild-type strain.35 A vaccine consisting of types 5 and 8 polysaccharide reduced S. aureus bacteremia by more than half in hemodialysis patients.36 The duration of protection was approximately 40 weeks after a single vaccination.
S. aureus exotoxins (e.g., TSST-1 and enterotoxins) act as superantigens that bind to host major histocompatibility complex (MHC) class II molecules and T cell receptors, resulting in clonal expansion and activation of some T cells. This activation triggers the release of numerous cytokines including interleukin (IL)-2, interferon-γ, and tumor necrosis factor (TNF).37 Induction of these cytokines results in systemic toxicity and joint damage. The stimulated T cells initially proliferate but later disappear, likely due to apoptosis, and result in immunosuppression.38 Internalized organisms that had been protected from this inflammatory response may cause fulminant or persistent infection. Mice injected with strains of S. aureus lacking TSST-1 and enterotoxins rarely develop arthritis; when arthritis is induced, it is much milder compared with arthritis in animals injected with the wild-type strain.37 Vaccination of mice with a mutated, recombinant form of enterotoxin A devoid of superantigen function was associated with a significant reduction in mortality.39
In response to bacterial infection of the joint space, the host releases a variety of cytokines and inflammatory mediators. Initially, IL-1β and IL-6 are released into the joint space, leading to an influx of inflammatory cells. These neutrophils and macrophages engulf invading bacteria and release additional cytokines including TNF, IL-1, IL-6, and IL-8. Blocking TNF with a monoclonal antibody and IL-1 with an IL-1 receptor antagonist inhibited leukocyte infiltration into the joint by 80% in a rabbit model of S. aureus–induced arthritis when the cytokine inhibitors were given simultaneously with S. aureus.40 When the same inhibitors were given 24 hours after infection, however, there was no effect on leukocyte infiltration, suggesting the crucial roles of TNF and IL-1 in the early stages of S. aureus–induced arthritis. Release of interferon-γ is associated with the influx of T cells, which occurs a few days after infection. In a mouse model of S. aureus septic arthritis, interferon-γ has been associated with a worsening of the severity of arthritis while protecting the animals from septicemia.41 The host’s early cytokine response may aid the clearance of organisms and limit infection in the host. A late cytokine response may amplify the destructiveness of an established infection.
Clinical Features
Acute bacterial arthritis is most commonly monoarticular. Polyarticular infection occurs in 5% to 8% of pediatric cases and in 10% to 19% of adult nongonococcal cases.42 The differential diagnosis of acute monoarthritis overlaps with many causes of polyarthritis because virtually any form of arthritis can initially manifest as a single swollen joint. The three main etiologies to consider when a patient presents with acute monoarticular arthritis are trauma, infection, and crystal-induced synovitis such as gout or pseudogout. Polyarticular septic arthritis is usually seen in patients with systemic inflammatory disorders such as the spondyloarthropathies, RA, systemic lupus erythematosus, and other connective tissue diseases or patients with overwhelming sepsis.15,43
Disseminated gonococcal infection occurs in 1% to 3% of patients infected with N. gonorrhoeae. Gonococcal arthritis is the most common cause of acute monoarthritis in sexually active young adults. In the preantibiotic era, gonococcal arthritis was a well-recognized illness in neonates. Disseminated gonococcal infection is three times more common in women than men. Women are more commonly affected because they are more likely to have asymptomatic and untreated primary infections. Bacterial dissemination has been associated with intrauterine devices and has occurred during menstruation, pregnancy, and pelvic operation.44
Patients with gonococcal joint disease typically present with one of two forms. The first form is characterized by fever, shaking chills, vesiculopustular skin lesions, tenosynovitis, and polyarthralgias. Blood cultures are frequently positive, whereas synovial fluid cultures are rarely positive. N. gonorrhoeae can be cultured from genital, rectal, and pharyngeal sites. Tenosynovitis of multiple tendons of the wrist, fingers, ankle, and toes is a unique feature of this form of disseminated gonococcal infection and distinguishes it from other forms of infectious arthritis. In the second form of gonococcal infection, patients have purulent arthritis, most commonly of the knee, wrist, or ankle, and more than one joint can be infected simultaneously. N. gonorrhoeae can frequently be cultured from the synovial fluid.45
The classic presentation of nongonococcal septic arthritis is the acute onset of pain, swelling, and decreased range of motion in a single joint. Large joints are affected most commonly. In adults, the knee is involved in more than 50% of cases; hip, ankle, and shoulder infections are less common.41 In infants and small children, the hip is more often involved.46 Patients with septic arthritis often have underlying illnesses and predispositions to infections. Many are immunocompromised; are intravenous drug abusers; have prosthetic joints; and have diseases such as neoplasia, renal failure, and RA. Table 109-3 lists the risk factors that predispose to septic arthritis.3,5,47
Table 109-3 Risk Factors for Development of Septic Arthritis
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Most patients with bacterial arthritis are febrile, although chills are unusual. Fever may be absent in elderly patients. In children, septic arthritis usually is accompanied by fever, malaise, poor appetite, irritability, and progressive reluctance to use the affected limb. Physical examination typically reveals warmth and tenderness of the affected joint, joint effusion, and limited active and passive range of motion. Septic arthritis among patients with RA has been a special challenge to clinicians because of the high incidence of infection and the poor outcome. Septic arthritis in patients with RA is associated with poor joint outcome and high mortality.42,48 In many cases, it is difficult to differentiate septic arthritis in a joint already affected by RA from rheumatoid flare. Whenever bacterial arthritis is suspected, the most important diagnostic procedure is arthrocentesis and examination of the synovial fluid. For joints that are deep and more difficult to aspirate, ultrasound-guided or fluoroscopy-guided needle aspiration should be done.
Diagnosis and Diagnostic Tests
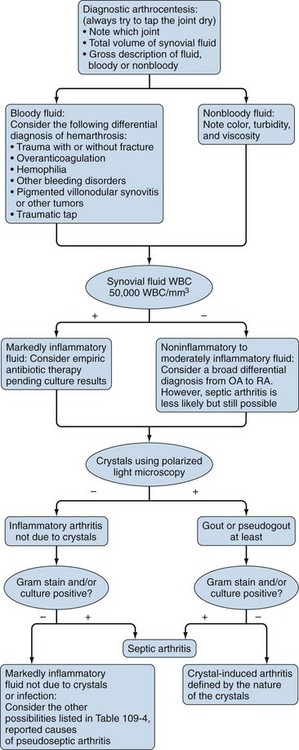
Arthrocentesis and synovial fluid analysis should be performed for all patients who present with an inflamed joint. Normal joints contain a small amount of synovial fluid that is clear, is highly viscous, and has few white blood cells (WBCs). The protein concentration is approximately one-third that of plasma, and the glucose concentration is similar to that of plasma. Infected synovial fluid is usually purulent with an elevated leukocyte count typically greater than 50,000 WBC/mm3 and often exceeding 100,000 WBC/mm3 with polymorphonuclear cell predominance. Synovial fluid levels of glucose, lactate dehydrogenase, and total protein have limited value in the diagnosis of septic arthritis. Although a low synovial fluid glucose (<40 mg/dL or less than half the serum glucose concentration) and an elevated lactate dehydrogenase suggest bacterial infection, they are not sufficiently sensitive or specific for the diagnosis.49 Figure 109-2 is an algorithm for synovial fluid analysis; Table 109-4 lists the differential diagnoses of septic arthritis and the known causes of pseudoseptic arthritis.50
Table 109-4 Differential Diagnosis of Septic Arthritis and Reported Causes of Pseudoseptic Arthritis*
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|