14 B Cells
Overview: B Cells and Humoral Immunity
B cells (Figure 14-1) are lymphocytes that recognize antigens through a molecule called the B cell receptor. The B cell receptor is composed of a surface immunoglobulin molecule, which recognizes the antigen, and two associated proteins, which transduce the signal. On encounter with its antigen, a B cell begins a process of activation leading to antibody secretion and memory formation regulated by interplay with antigen-activated T cells, dendritic cells, soluble factors, and in some cases follicular dendritic cells. Both T and B lymphocytes can differentiate from naïve to memory cells, but only B cells have the capacity to fine tune their antigen receptor structure to increase its specificity and affinity, giving rise to more effective antibodies. Beyond immunoglobulin secretion, B cells regulate the immune response by cytokine secretion and antigen presentation to T cells in the context of class II molecules.
Immunoglobulins: Structure and Function
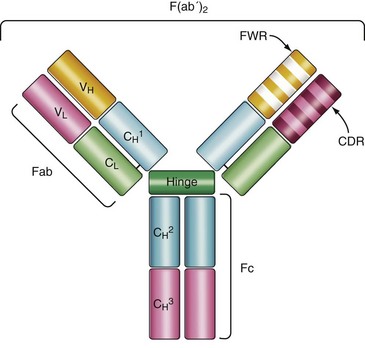
Structurally, Igs, also referred to as antibodies, are composed of four polypeptide chains: two identical light (L)-chains with a molecular weight of approximately 25 kDa and two identical heavy (H)-chains of 50 to 65 kDa. Each of the chains contains a folding motif that is highly conserved among proteins of the immune system, the “Ig domain.” These domains constitute the backbone of the Ig molecule and make for the interface along which the polypeptide chains pair (Figure 14-2). The quaternary structure of an immunoglobulin molecule assumes a Y-shaped conformation, which contains two functional moieties: two identical antigen-binding regions or variable regions, which are the arms of the “Y,” and a constant region, which is the base of the “Y.”1
This definition of functional moieties derives from early studies analyzing proteolytic fragments of Ig molecules. Cleavage with papain generates two identical fragments that retain antigen-binding capacity and hence are named Fab, as well as a distinct crystallizable fragment Fc that mediates immune effector functions but is unable to interact with antigen.2
The antigen-binding regions are formed by pairing of the variable domain of the L-chain (VL) to the variable domain of the H-chain (VH). In contrast to the rest of the molecule, there is a great diversity in the amino acid sequence of the variable domains, which allows for a broad repertoire of antibody molecules that can recognize a wide array of antigens. Within the variable region of the Ig molecule are discrete regions, known as complementary determining regions (CDRs) that make direct contact with antigen. The amino acid sequences of the CDR are highly variable and are flanked by more conserved amino acid sequences called framework regions. The H- and L-chain molecules each contain three CDRs and four framework regions (see Figure 14-2). The minimal antigenic determinant recognized by the H and L CDR is known as an epitope, which may be a continuous or discontinuous region on a protein, carbohydrate, lipid, or nucleic acid. The presence of two identical variable regions in a single Ig molecule confers the capacity to interact with repetitive antigenic determinants present in multivalent antigens (i.e., polysaccharides) or two separate antigen molecules containing the same antigenic determinant.1
Immunoglobulin Constant Region
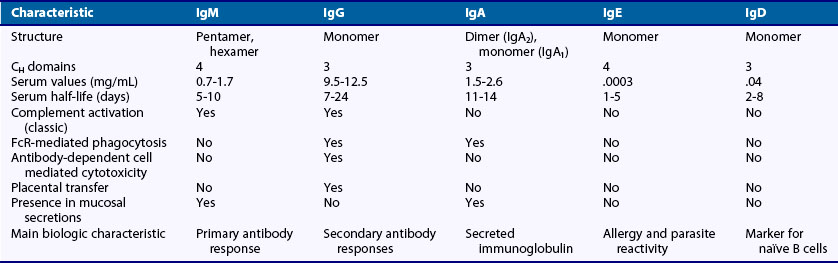
The specific binding interactions that occur between the Ig variable region and antigen may be sufficient to block microbial infectivity or neutralize toxins. However, the ability to eliminate pathogens is mediated by the Fc portion of the molecule. The Fc regions of antigen-antibody complexes are made accessible to serum factors that comprise the complement cascade or to cytotoxic and phagocytic cells that mediate the destruction and removal of pathogens. In mice and humans, there are five different types of H-chain constant regions, or isotypes, designated IgM (µ), IgD (δ), IgG (γ), IgA (α), and IgE (ε)3; each is encoded by a distinct constant region gene segment present in the H-chain locus of chromosome 4 in humans or 12 in mice. Each isotype is capable of specific effector functions, and each cellular receptor for Ig initiates a distinct intracellular signaling cascade. The number of CH domains, presence of a hinge region to increase flexibility between Fab regions, serum half-life, ability to form polymers, complement activation, and Fc receptor binding vary among isotypes. Characteristics of the different Ig H-chain isotypes are presented in Table 14-1.1,3,4 These isotypes may also differ in the intracellular signaling they initiate when bound by antigen in their membrane-associated form on the B cell. It should be noted that the interplay between antibodies and the cells that bear the Fc receptors extends beyond pathogen clearance and shapes the immune response by mediating activation or inhibition of specific cell types5 and by mediating cell death.6
Immunoglobulin M
IgM is the first isotype expressed in developing B cells and the first antibody secreted during a primary immune response. It is found predominantly in serum but is also present in mucosal secretions and breast milk. Because the process that increases antibody affinity for a particular antigen (affinity maturation) has not yet been initiated during the early stages of a primary immune response, IgM antibodies usually exhibit low affinity. Their low affinity is balanced by the fact that most of the secreted IgM exists in pentameric form, generating multiple binding sites, providing high avidity for antigen and assisting the binding of large, multimeric antigens. IgM also exists as a monomer and a hexamer, but only the pentameric form is linked by the polypeptide called joining (J)-chain. The J-chain allows the active transport of IgM to mucosal secretions.7
Many of the biologic functions of IgM are mediated by its ability to activate the classic complement pathway.1 The complement cascade is composed of a series of enzymes that, on activation, mediate the removal and lysis of invading organisms. Deposition of antibody molecules or complement components on the surface of the antigen assists phagocytosis. Proteins such as antibody and complement that enhance phagocytosis are called opsonins. Once the complement cascade has been activated, monocytes, macrophages, or neutrophils engulf opsonized particles through specific receptors present on phagocytic cells such as CD21, which recognizes fragments of the C3 complement component. Activation of the complement pathway also results in the generation of the membrane attack complex, which is composed of late complement components and directly lyses C3-opsonized pathogens. Because activation of the classic complement pathway requires Fc regions to be spatially close, but also exposed, multimeric IgM is a potent activator of the classic complement pathway once it has bound its antigen. For example, hexameric IgM is between 20 and 100 times more potent as an inducer of complement activation than monomeric IgM.8
Immunoglobulin G
In humans there are four subclasses of IgG: IgG1, IgG2, IgG3, and IgG4. IgG1 and IgG3 arise in response to viral and protein antigens. IgG2 is the main antibody present in response to polysaccharide antigens, and IgG4 participates in responses to nematodes and is observed in chronic antigenic stimulation.9
All IgG subclasses exist as monomers and have a high structural similarity; however, minor differences make for distinct biologic effects. IgG3 and IgG1 are potent activators of the classic complement pathway, and IgG2 can initiate the alternative complement pathway (see Chapter 23).
All IgG subclasses engage specific Fc gamma receptors (FcγRs) present on dendritic cells, macrophages, neutrophils, and NK cells. The FcγRs on phagocytic cells, when cross-linked, mediate the removal of immune complexes from circulation and initiate antibody-dependent, cell-mediated cytotoxicity resulting in the release of granules that contain perforin, a pore-forming protein, and enzymes known as granzymes that induce programmed cell death (apoptosis) of target cells.10,11 FcγR engagement also allows the internalization and subsequent presentation of antigens in the context of MHC class II molecules.
Because IgG antibodies are the only ones that cross the placental barrier, they are critical for the survival of newborns. The transport of IgG from the maternal circulation into the fetal blood supply is mediated by the FcRn receptor.12 FcRn is also responsible for the long half-life of IgG in serum by blocking IgG catabolism.13
Immunoglobulin A
Despite its relative low concentration in serum, more IgA is produced than all other isotypes combined. Most IgA exists as secretory IgA (SIgA) in mucosal cavities and in milk and colostrum, and only a small fraction is present in serum. Two subclasses of IgA exist in humans: IgA1 and IgA2. IgA1 is mainly produced as a monomer. In contrast, polymeric IgA2 is produced along mucosal surfaces.14
Polymeric IgA exists mainly as a dimer and includes a J-chain (the same chain that links pentameric IgM). It is captured by the polymeric immunoglobulin receptor (pIgR) that is expressed on the basolateral surface of the epithelial cells and then transcytosed to the apical side. Release of IgA into mucosal secretions requires cleavage of the pIgR; a fragment known as the secretory component (SC) remains associated with secreted IgA and protects it from the action of proteases and increases its solubility in mucus, where it neutralizes toxins and inhibits the adherence of secreted IgA-coated microorganisms to the mucosal surface.15
People with IgA deficiency have reduced levels of both serum and secreted IgA and are prone to respiratory tract and diarrheal infections, as well as an increased incidence of autoimmune disorders.16 FcαR, present in the surface of neutrophils and macrophages, has been suggested to play a regulatory role in the immune system; it is not clear if the autoimmune manifestations in IgA-deficient patients are a consequence of the absence of the regulatory loop played by engagement of FcαR.17
Immunoglobulin E
IgE is involved in protection against parasitic infections but also triggers immune responses associated with allergic reactions. Only a small amount of IgE is detectable in serum, where it exists as a monomer.18 Mast cells and basophils express a high-affinity IgE Fc receptor (FcεRI) that binds free IgE. Cross-linking of the FcεR by antigen binding to the IgE induces degranulation and release of histamine, proteases, lipid mediators such as prostaglandin D2 and leukotrienes, many of which are associated with anaphylaxis.
Immunoglobulin D
The role of IgD in the humoral response has been the subject of multiple speculations. IgD is found predominantly as a membrane immunoglobulin on the surface of mature naïve B cells. Soluble IgD is scarce in serum; however, IgD-producing plasma cells are found in tonsils and tissue associated with the respiratory tract. High levels of secreted IgD can be found in individuals with immunodeficiencies.19
Light Chains
There are two distinct L-chain polypeptides, designated kappa (κ) and lambda (λ). L-chains contain a variable and a single constant domain. Even though there are two L-chain isotypes, there is no known function associated with the L-chain constant region. The κ chain is used more often than the λ chain in human (65%) and mouse (95%) Ig molecules.20
Immunoglobulin Variable Region
The recognition of a virtually unlimited number of antigens requires a mechanism to generate Ig molecules with similarly broad specificities. The molecular basis of this process has been known for several years.21 First, the Ig molecule is composed of both heavy and light chains. These chains are encoded within distinct genetic loci residing on separate chromosomes; the H-chain locus is on human chromosome 14,22 the κ-chain locus is on chromosome 2, and the λ-chain locus is on chromosome 22.23
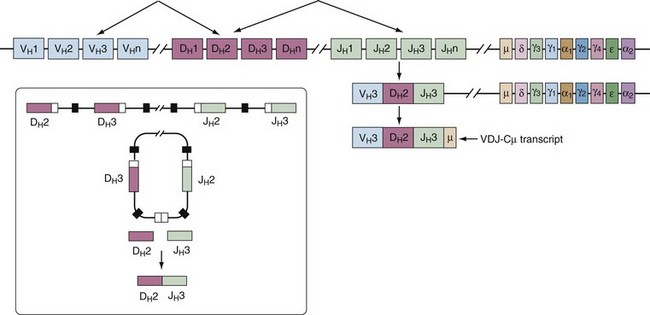
Within each locus, gene segments encode both the variable and constant regions of the Ig molecule. The H-chain variable region is encoded by a variable (VH), diversity (DH), and a joining (JH) segment. The L-chain is encoded by either Vκ and Jκ or Vλ and Jλ segments; it does not contain D segments. The human H-chain locus contains from 38 to 46 VH, 23 DH, and 9 JH functional genes (these numbers represent a typical haplotype, but vary among individuals). The κ-chain locus contains approximately 31 to 35 Vκ genes and 5 Jκ functional genes; the λ-chain locus contains 29 to 32 Vλ genes and 4 or 5 Jλ functional genes.24
Generation of Immunoglobulin Diversity
In a developing B cell, different VH, DH, and JH or VL and JL gene segments are randomly combined to generate a large number of different Ig molecules (Figure 14-3). This process, known as V(D)J recombination, occurs in the primary lymphoid tissue, in the absence of antigen stimulation and must be successful to continue with B cell maturation. Here, we present the molecular process, and later the functional and developmental consequences are discussed (see B Cell Development).
V(D)J recombination happens sequentially, beginning with the joining of one DH segment to one JH. Following this, a VH segment will be targeted to the rearranged DHJH fragment. The absence of an in-frame recombination leads to recombination of the second allele. Light chain recombination also occurs stepwise. First, the kappa locus is rearranged; in absence of a productive κ-chain rearrangement, the lambda locus undergoes recombination.25
The recombination machinery is composed of specific enzymes including Recombination-Activating Gene 1 (RAG-1) and the Recombination-Activating Gene 2 (RAG-2). The complex recognizes recombination signal sequences (RSS) that flank the V, D, and J gene segments. These highly conserved sequences are composed of a palindromic heptamer (7 base pairs) followed by deoxyribonucleic acid (DNA) spacers that are 12 or 23 base pairs in length and an AT-rich nonamer.26 Once the complex recognizes its target, it generates double-stranded DNA (dsDNA) breaks at the RSS sites. Next, the cellular DNA repair complex recognizes and joins the cleaved segments.
B Cell Development
B cells, as all cells in the hemopoietic lineage, begin with the differentiation of noncommitted, undifferentiated CD34+ hematopoietic stem cells to lymphopoietic precursors with restricted lineage potential. These cells, known as common lymphoid progenitors (CLP), have the potential to give rise to NK, T, and B cells. Early B cell progenitors begin to express genes for DNA rearrangement, as well as B cell program transcription factors. Multiple transcription factors act in concert, but Ikaros, E2A, EBF, and Pax5 appear to be the most important in B cell development.27 Pax5 is considered the master transcriptional control for B cells because it is induced in early stages of B cell commitment and plays a dual role by repressing genes required for differentiation to the myelomonocytic lineage and activating B cell–specific genes such as Ig genes, CD19, and signaling molecules.28
Niches of Human B Cell Lymphopoiesis
The development of undifferentiated hematopoietic stem cells (CD34+) into mature B cells begins in the first weeks of uterine life. By the eighth gestational week, early B cell precursors can be identified in the fetal liver and omentum. From gestational week 34 and through adulthood, the bone marrow is the primary site of lymphopoiesis.29
It has been unequivocally established that there are differences between the B cells that originate during fetal and adult lymphopoiesis in mice, and it is becoming clear that these differences extrapolate to human lymphopoiesis as well. B cell precursors are susceptible to estrogen, and the maturation of maternal B cells is arrested in the pro–B cell stage during pregnancy; in contrast, fetal B cell precursors lack estrogen receptors and consequently are unaffected by exposure to hormones.30 B cells originating during prenatal life have a bias in the usage of DH and JH gene segments, and this, along with reduced expression of the enzyme TdT, leads to a more restricted repertoire with shorter CDR3s.31
Whether during fetal or adult lymphopoiesis, the maturation of B cells from pluripotential progenitors is contingent on the presence of stromal cells that provide both contact-dependent and soluble signals. Although the nature of the interactions provided by the stromal cells to create a lymphopoiesis-permissive environment is still largely unknown, they include both survival and proliferative signals. The stromal-derived factor-1 (SDF-1) is required for the homing of the early B cell precursors to sites of lymphopoiesis but also promotes differentiation.32 Interactions between VCAM-1 on the membrane of the stromal cells and its counterpart, VCAM-4, on early B cell progenitors are required for B cell differentiation. The molecules IL-7, IL-3, and the Flt3 ligand promote B cell lymphopoiesis, although IL-7 appears to be dispensable for human B cell development. Matrix molecules in the microenvironment such as heparan sulfate proteoglycan are assumed to “trap” critical soluble factors.33
B Cell Ontogeny
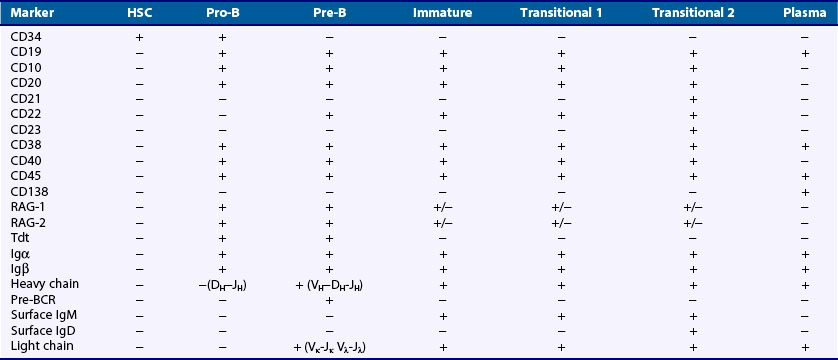
The stages of B cell development are defined by the state of Ig gene rearrangement and the expression of intracellular and surface proteins. The nomenclature and classification of particular stages vary slightly among different laboratories working in this field. For simplicity, we have divided the stages of B cell lymphopoiesis into early B cell progenitors, pro-B, pre-B, immature, transitional, and mature naïve cell (Table 14-2).
Pro-B Cells
This stage is defined by the rearrangement of the heavy chain gene segments and synthesis of a µ-polypeptide. Pro-B cells are dependent on interactions with endothelial cells present in the stroma. The VLA-4 integrin receptor and CD44, which both mediate adhesion to stromal cells, are highly expressed at this stage and are believed to be important for continued development.34 Pro-B cells also express high levels of Bcl-2, a molecule that protects cells from apoptosis. At the onset of the pro-B cell stage, the variable gene segments of both H- and L-chain loci are in the unrearranged germline configuration but accessible to the recombination machinery. A DH gene segment on one H-chain chromosome rearranges with a JH gene segment residing on the same chromosome, often with the inclusion of nontemplate nucleotides at the junction of these two segments. Next, a VH gene rearranges to the DHJH gene fragment. Completion of VHDHJH gene rearrangement leads to the generation of an H-chain transcript that also contains the IgM constant region (Cµ), which is the constant region gene most proximal to the variable region genes on the chromosome (see Figure 14-3).
The generation of a µ-polypeptide and its subsequent expression on the surface of the cell, together with a surrogate light chain formed by the λ5 and Vpre-B polypeptides as well as the Igα/Igβ dimer, a complex known as the pre-B cell receptor (pre-BCR), marks the end of this phase of gene recombination. This constitutes a critical developmental checkpoint and the entrance to the next developmental stage known as the pre-B cell.35 The requirement for a pre-BCR complex ensures that B cells without a productive H-chain will not undergo further differentiation.
Pre-B Cells
At the onset of the pre-B cell stage, the expression of the pre-BCR induces a proliferative burst that generates daughter cells with the same heavy chain and potential for multiple specificities within daughter cells, each of which may produce a different light chain. It is unknown if expression of the pre-BCR and tonic signaling is enough to trigger these events36 or if a ligand must engage the pre-BCR.37,38 Either way, targeted disruption of genes encoding the pre-BCR complex such as the IgM transmembrane constant region domain, λ5, or the Igα and Igβ accessory molecules results in a profound decrease in developing B cells. Also, defects in the adapter molecule BLNK, λ5, or the tyrosine kinase Btk lead to a serious impairment in pre-B cell maturation.
The expression of the pre-BCR is transient. After the proliferative burst, the µ-heavy chain is present only in the cytoplasm as the pre-B cell rearranges an L-chain. The general rearrangement process is similar to V(D)J rearrangement and is dependent on Rag1/2 expression. Because TdT is not expressed in this stage, L-chains do not usually contain N sequences at the VLJL junction. At the end of this process, pairing of the newly minted light chain with the µ-heavy chain leads to the surface expression of an IgM molecule, complexed with Igα and Igβ, to form the B cell receptor (BCR). It is believed that surface expression of the BCR on immature B cells transduces signals that enforce allelic exclusion at the L-chain locus and downregulate expression of the RAG genes. This immature B cell has completed the gene rearrangement process and is now subject to repertoire selection.39
Peripheral B Cell Subsets
Transitional B Cells
Transitional cells are the last B cell subpopulation that expresses the developmental marker CD24. At this stage B cells begin to express surface IgD, which harbors the same specificity as the IgM because the IgD heavy chain is encoded by the same VDJ fragments as IgM but expresses the Cδ instead of the Cµ domain. It is the expression of IgD that separates transitional cells into two different maturation stages. Transitional 1 (T1) B cells that do not express IgD are the recent bone marrow emigrants, and transitional 2 (T2) B cells that begin to express IgD represent the subsequent maturational stage.40 The existence and functional characteristics of a third transitional stage (T3) are still debated.
Transitional cells constitute a stage subject to multiple regulatory processes. First, transitional cells must compete with naïve B cells already present in the periphery for a developmental niche. Transitional B cells are extremely dependent on a B cell survival factor known as B cell–activating factor of the tumor necrosis factor family (BAFF or BLyS). In its absence, B cell development does not progress beyond the T1 stage.41 Second, T1 transitional cells are still highly susceptible to tolerance induction following BCR cross-linking. In T1 cells cross-linking of the BCR ex vivo has been shown to lead to cell death, whereas T2 cells respond to BCR cross-linking by proliferation and differentiation to the mature naïve B cell stage.
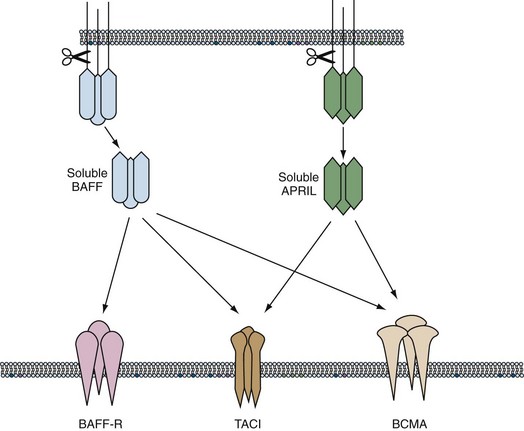
BAFF Family of Cytokines
The cytokine milieu surrounding the B cell is diverse and spatially and temporarily regulated. Although B cells are modulated by multiple cytokines, in recent years two members of the TNF family, BAFF and APRIL, have emerged as key survival factors, particularly at two regulatory points in development and differentiation: the transition from an immature to a naïve B cell in the periphery and the survival of the newly produced plasma cells. BAFF (B cell–activating factor, BLyS) and APRIL (A proliferation-inducing ligand) are proteins produced by cells that take part in the innate response such as macrophages and dendritic cells, as well as stromal cells, and are present as membrane-bound proteins or soluble trimers. They have three known receptors (BAFF-R, TACI, and BCMA) that are present on the membrane of B cells from the T2 stage to their final differentiation to plasma cells. BAFF binds the three receptors, whereas APRIL binds only TACI and BCMA. BAFF induces survival and activation of B cells when bound to BAFF-R, whereas BAFF signaling through TACI decreases the size of the B cell pool. APRIL does not participate in B cell homeostasis but seems critical to the survival of plasmablasts in the bone marrow.42
Enhanced survival and activation of autoreactive B cells have been demonstrated in mice that overexpress BAFF.43 An increase in serum levels of BAFF has been observed in some patients with lupus, rheumatoid arthritis, and Sjögren’s syndrome and is thought to contribute to pathogenesis because autoreactive B cells have a survival advantage in the presence of excess BAFF44 (Figure 14-4).
Naïve B Cells
The final stages of maturation that occur in the spleen and give rise to the naïve B cell subset have not been fully elucidated, but the prevailing theory is that T2 B cells give rise to the circulating mature naïve cell population. In the mouse, two populations of phenotypically and functionally different naïve B cells are recognized: follicular and marginal zone B cells. Human naïve B cells comprise 60% to 70% of the circulating B cell repertoire and populate the spleen and lymph nodes. They include the equivalent of mouse follicular B cells and represent the circulating, nonantigen exposed B cell subpopulation characterized by surface IgM and IgD expression, lack of CD27, and the presence of the membrane transporter ABC.45 There is also a population in blood of IgM+, CD27+ B cells that have been likened to marginal zone B cells, which do not recirculate in the mouse.46
Marginal Zone B Cells
In the human spleen, the structural definition of the marginal zone does not correspond exactly to the area surrounding B cell follicles. However, there is a population with the functional characteristics of mouse MZ B cells: low activation thresholds, highly responsive to polysaccharide antigens and with a clear surface phenotype, can be differentiated. These cells, which are sometimes named MZ-like or unswitched memory, are not restricted to the human spleen but are found circulating in the peripheral blood, as well as in the lymph nodes, tonsils, and Peyer’s patches.46,47 They are defined as IgM+, IgD+, CD27+, CD21+, and CD1c+.47
Sites of B Cell Homing and Activation
After the immature B cell stage, B cells home to secondary lymphoid organs, which contain the microenvironment and architecture necessary for the retention and activation of B cells. These organs include the spleen and lymph nodes, as well as lymphoid structures in mucosal tissue (e.g., Peyer’s patches, appendix, tonsils). The secondary lymphoid tissue is adapted to trap circulating antigen and expose the B cells to it and to provide interactions with T cells and other co-stimulatory cells. Peripheral lymphoid tissue contains specialized antigen-presenting cells known as dendritic cells. In the Peyer’s patches of the intestines, foreign antigen is taken up in specialized epithelial cells known as M cells. Even though peripheral lymphoid tissues vary in structure and cellular organization, they all possess antigen-presenting cells and B cell–containing follicles surrounded by T cell–rich zones.49 As explained in the following section, antigen, T cells, and dendritic cells are required for B cell activation and differentiation into Ig-secreting plasma cells or memory B cells.
Circulation and Homing
The entry, retention, and recirculation of B cells through secondary lymphoid organs depend on both adhesion molecules and chemokine receptors.50,51 First, expression of LFA-1 and VLA-4 is required for entry into the lymphoid tissue. Then the chemokine receptors CXCR5 and CCR7 direct localization within the tissue. The CXCR5 molecule is expressed on all mature B cells and mediates B cell migration to follicles in response to the chemokine CXCL13, which is produced by follicular stromal cells. These cells, in turn, are regulated by lymphotoxin-α made by B cells. In the follicle, the B cells scan for antigen, making contact with potential antigen-bearing cells such as follicular dendritic cells (FDC), subcapsular macrophages, and dendritic cells. If the B cell does not encounter a cognate antigen, it will exit the lymphoid organ through the efferent lymphatics in response to the molecule sphingosine-1-phosphate (S1P). Neutralization of S1P leads to sequestration of B cells in lymphoid organs.52
The interplay of chemokine expression and the induction of chemokine receptors play an important role in the germinal center response. The chemokine CXCL12 retains centroblasts in the dark zone during the process of somatic hypermutation and isotype class switching. CXCL13 regulates migration to the light zone, where survival and selection events are mediated by interactions with CXCR5-expressing follicular helper T cells and FDCs.53 Later, CXCL12 promotes the migration of plasmablasts to the bone marrow, where they undergo further development into long-lived plasma cells.
Mucosa-Associated Compartments
Within the mucosal tissue, the sites of induction of immune responses are distinct from the site where the effector cells reside. There are two main sites for the induction of an immune response. The first is the mucosa-associated lymphoid tissue (MALT) that includes Peyer’s patches, nasopharynx-associated tissue, and isolated lymphoid follicles, where exogenous antigen is displayed by specialized M cells that transport antigen to the follicle. The second site of induction includes mucosa-draining lymphoid nodes such as the mesenteric and cervical lymph nodes.53
B cells reach these sites through the systemic circulation. Once they are stimulated by antigen and induced to differentiate, they home to the effector sites in the intestinal and respiratory lamina propria, where they differentiate into plasma cells and produce antibody mainly of the IgA isotype. An interesting characteristic of the plasma cells induced in the mucosal compartments is their selective homing to mucosal effector sites. Nasal activation leads to IgA-secreting cells with high levels of CCR10 and α4β1-integrins that home to the respiratory and genitourinary tracts in response to their ligands, CCL28 and VCAM-1. Migration to the intestinal lamina propria, in contrast, seems to be dependent on orally induced activation and subsequent expression on B cells of the chemokine receptor CCR9 and integrins that bind to CCL25 and MADCAM1/ VCAM-1, respectively.54
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree