53 Synovial Fluid Analyses, Synovial Biopsy, and Synovial Pathology
![]() Video available on the Expert Consult Premium Edition website.
Video available on the Expert Consult Premium Edition website.
Synovial Fluid Analysis
Synovial Fluid in Health
Under normal conditions, a small volume of synovial fluid is present in each joint, forming a thin interface between the surfaces of the articular cartilage, and providing for friction-free movement of these surfaces. In a large joint such as the knee, the volume of synovial fluid is estimated to be less than 5 mL. Moreover, intra-articular pressure is typically subatmospheric. Compositionally, normal synovial fluid is an ultrafiltrate of plasma to which proteins and proteoglycans are added by fibroblast-like synoviocytes in the lining layer. Most of the small-molecular-weight solutes such as oxygen, carbon dioxide, lactate, urea, creatinine, and glucose diffuse freely through the fenestrated endothelium of the synovium and are normally present at levels comparable with those of plasma. Evidence for active transport of glucose has been found. The total protein concentration of normal synovial fluid is 1.3 g/dL. The concentration of individual plasma proteins is inversely proportional to the molecular size, with small proteins such as albumin present at approximately 50% of plasma levels, and large proteins such as fibrinogen, macroglobulins, and immunoglobulins present at low levels. It should be noted that in contrast to this selective entry on the basis of size, clearance of synovial fluid proteins through the synovial lymphatics is unrestricted by size. Hyaluronan is the major proteoglycan synthesized by synovial cells and secreted into synovial fluid. Hyaluronan is highly polymerized and reaches molecular weights exceeding one million daltons, giving this fluid its characteristic viscosity. The hyaluronan also acts to retain small molecules in the synovial fluid. The lubricating capacity of the synovial fluid is attributed to a glycoprotein called lubricin.1 This molecule has been fully characterized on the basis of the study of individuals with mutations of the PRG4 gene, which encodes for its production.2 These mutations result in an autosomal recessive loss-of-function disorder called the camptodactyly–arthropathy–coxa vara–pericarditis syndrome, which features a progressive, noninflammatory arthropathy characterized by severe cartilage destruction associated with proliferation of synovial lining cells. The role of lubricin in maintaining the health of the cartilage has been further demonstrated in a murine knockout model.3
Arthrocentesis
Most peripheral joints are readily accessible for diagnostic arthrocentesis, and the procedure can be performed in almost any ambulatory care setting equipped for sterile procedures. Joints that are less accessible because of a deeper location, such as the hip, may require an imaging technique that uses fluoroscopy or ultrasound to guide the needle and ensure accurate placement. Details of techniques used for arthrocentesis are described in Chapter 54. Because the ease with which joint fluid is aspirated depends on the gauge of the needle that is used, it is important to attempt arthrocentesis with a needle of adequate gauge, particularly in the larger joints. Moreover, high suction gradients created by large syringes should be avoided, because they may actually reduce the capacity to successfully aspirate synovial fluid. Difficulty in aspirating synovial fluid may relate to a number of intra-articular factors, including viscosity, the presence of debris such as rice bodies, and loculation of fluid into inaccessible areas. Instillation of a small amount of sterile saline may assist in obtaining enough fluid for culture in situations where infection is highly suspected, yet direct aspiration is difficult.
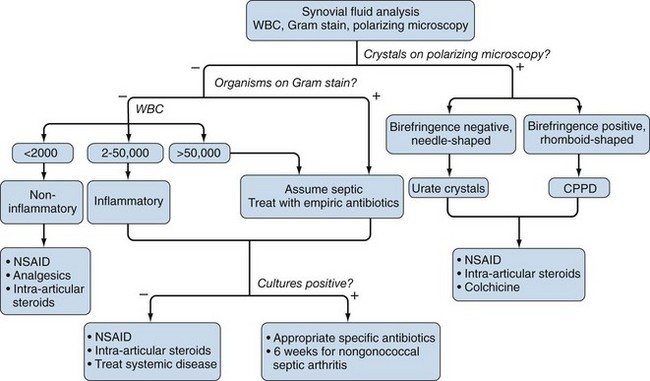
Once obtained, it is important to analyze aspirated synovial fluid samples as quickly as possible to avoid spurious results. In particular, leukocyte count and differential ideally should be performed on fresh specimens. If the specimen cannot be analyzed quickly and short-term storage is needed, the specimen should be kept at 4° C, and an aliquot preferably placed in ethylenediaminetetraacetic acid (EDTA) to prevent clotting. Delays in analysis beyond 48 hours should be avoided. A simplified algorithm for analyzing synovial fluid samples is shown in Figure 53-1.
Leukocyte Count
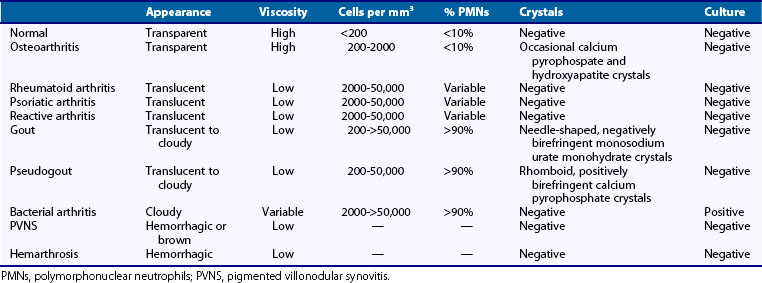
Analysis of leukocyte counts and cytology provide important diagnostic information regarding the cause of a synovial effusion (Table 53-1). A fresh specimen should be placed in a heparinized tube for rapid analysis, and if the fluid is particularly viscous, it may need to be diluted in normal saline before counting. Normal synovial fluid contains fewer than 180 nucleated cells/mm3, most of which originate as desquamated synovial lining cells. The leukocyte count broadly classifies synovial fluids as noninflammatory (<2000 cells/mm3), inflammatory (2000 to 50,000 cells/mm3), and septic (>50,000 cells/mm3). It should be kept in mind that these definitions provide broad guidelines to help narrow the differential diagnosis rather than representing inherent biologic properties of the fluid.
The most common causes of noninflammatory synovial fluids are mechanical derangements of the joint and osteoarthritis. Other causes include endocrinopathies such as acromegaly and hyperparathyroidism; inherited disorders such as ochronosis, hemochromatosis (which can also present with hemarthrosis), Ehlers-Danlos syndrome, Wilson’s disease, and Gaucher’s disease; acquired disorders such as Paget’s disease, avascular necrosis, and osteochondirits dissecans; and an uncommon condition called intermittent hydrarthrosis, in which joints become effused in a cyclic manner. At the other extreme, leukocyte counts of 50,000 to 300,000 cells/mm3 are most commonly associated with septic arthritis and should prompt the clinician to empirically treat the individual as such until this diagnosis is excluded with a high degree of certainty, which typically requires definitive culture results, and possibly repeat aspiration. It should be added that leukocyte counts exceeding 50,000 cells/mm3 are not uncommonly seen in acute crystal-induced arthritis, particularly gout. Inflammatory cell counts between 3000 and 50,000 cells/mm3 are seen in a wide spectrum of articular disorders, including many cases of septic arthritis. Thus most patients with acute attacks of gout and pseudogout, active RA, reactive arthritis, and psoriatic arthritis, as well as patients with gonococcal arthritis and other nonpyogenic forms of septic arthritis, will typically present with synovial fluid cell counts in this range (see Table 53-1).
Synovial Fluid Cytology
Characterization of synovial fluid leukocytes is best achieved by staining a dried smear of the fluid. Wright stain is most commonly used for this purpose. The phenotype and morphology of the leukocytes can then be assessed under high power using oil immersion. Septic range synovial fluid containing more than 50,000 cells/mm3 is almost always associated with a high preponderance of polymorphonuclear leukocytes, often greater than 90%. Monocytes and lymphocytes predominate in the synovial fluid of patients with viral arthritis, lupus, and other connective tissue diseases. Synovial fluid samples from patients with active RA, reactive arthritis, psoriatic arthritis, and acute attacks of crystal-induced arthritis typically demonstrate a preponderance of polymorphonuclear leukocytes, although early RA fluids may have a low leukocyte count with primarily mononuclear cells. The presence of large numbers of “ragocytes,” which are granulocytes that have phagocytized immune complexes, is associated with active RA, and their presence may indicate an unfavorable prognosis in this disease.4 Reiter’s cells represent cytophagocytic mononuclear cells that have phagocytized apoptotic polymorphonuclear leukocytes, this possibly representing a pathway by which autolysis and release of damaging mediators from the latter cells are avoided.5 The presence of Reiter’s cells is not specific for reactive arthritis, nor indeed for spondyloarthropathies in general. Occasionally, eosinophils will be seen to predominate in the synovial fluid. This may be associated with parasitic infection, urticaria, or hypereosinophilic syndrome. It has been suggested that cytocentrifugation of synovial fluid is the optimum method for performing cytopathology, although the cost-effectiveness of this technique is questionable in most clinical settings.
Wet Smear Analysis by Polarized Microscopy
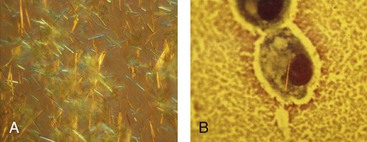
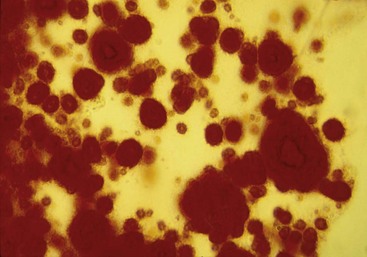
Identification of crystals in synovial fluid is greatly facilitated by detailed examination of the specimen, under both low and high power, using the approach previously described. A combination of morphology and birefringence serves to identify the crystals. Monosodium urate (MSU) crystals, as shown in Figure 53-2, are the easiest to identify because the crystal load is typically high during an acute attack of gout. A good degree of concordance between laboratories in the identification of MSU crystals has been shown.6–8 These crystals appear as strongly negatively birefringent needle-shaped objects, many of which are intracellular, having been phagocytized by synovial fluid leukocytes. In contrast, calcium pyrophosphate dihydrate (CPPD) crystals seen during attacks of pseudogout tend to be smaller, rhomboid-shaped objects that are weakly positively birefringent, as shown in Figure 53-3. Because the CPPD crystal load during an attack of pseudogout tends to be relatively low, and because CPPD crystals are only weakly birefringent, it is important to examine all areas of the specimen on the microscope slide, and possibly to prepare a second wet mount to exclude or confirm this diagnosis. Concordance between laboratories in the recognition of CPPD has been shown to be substantially lower than in the case of MSU crystals.6–8 A particularly challenging situation arises when intracellular crystals cannot be identified, yet birefringent extracellular objects resembling crystals are seen scattered throughout the slide. This may be caused by powder from gloves or dirt on the slides.
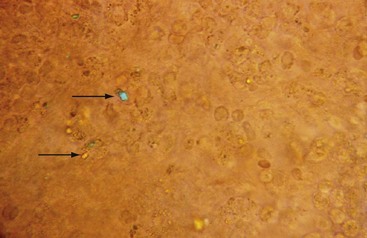
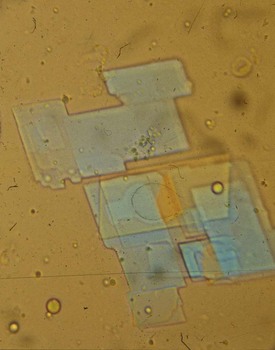
Deposits of hydroxyapatite or basic calcium phosphate are present within the joint and in periarticular locations such as around the shoulder area, and are associated with osteoarthritis. These crystals have been incriminated in a particularly destructive syndrome that has been named Milwaukee shoulder.9 Hydroxyapatite can be detected in synovial fluid, but because these crystals are generally nonbirefringent, it is not possible to detect them by polarized microscopy. A useful and rapid method with which to detect hydroxyapatite and other calcium-containing crystals such as octacalcium and tricalcium phosphate is to stain the fluid with alizarin red S stain and look for clumps of crystals under routine light microscopy (Figure 53-4). These crystals have also been identified using electron microscopy, although this method is rarely available to the practicing clinician.
Synovial cholesterol crystals appear as flat, plate-like structures with notched corners (Figure 53-5), and lipid crystals have the appearance of Maltese crosses. Both can be strongly birefringent, both negatively and positively. Corticosteroid crystals can be highly birefringent and mimic urate or CPPD crystals. Large amounts of lipid in the synovial fluid can be visible on gross examination. The significance of these crystals in synovial fluid is unclear, but it is unlikely that they are pathogenic in most cases.
Detection of Microorganisms by Gram Stain, Culture, and Polymerase Chain Reaction Analysis of Synovial Fluid
A Gram stain performed on fresh synovial fluid will identify an organism in an estimated 50% of cases of septic arthritis,10 with highest sensitivity for gram-positive organisms. Moreover, the specificity of a positive Gram stain approaches 100%. Clearly this indicates that the positive predictive value for the Gram stain is very high, and that the negative predictive value is substantially lower. The gold standard for diagnosing septic arthritis remains bacteriologic culture, which has a sensitivity of 75% to 95% and a specificity of 90% in cases of nongonococcal septic arthritis.11,12 It has been shown that the use of blood culture bottles further increases the yield of positive synovial cultures.13 It is important to note that bacteriologic cultures are the only studies that provide a guide for specific antimicrobial therapy. Because the sensitivity of bacteriologic cultures declines dramatically after antibiotic therapy is instituted, it is important that the clinician perform arthrocentesis before any antibiotics are administered. Cultures should be performed even when uric acid or other crystals are demonstrated in the synovial fluid, because it has been shown that gout and septic arthritis can coexist.14 In the case of gonococcal arthritis, the sensitivity of bacteriologic culture, even if performed on a sample collected using appropriate media, is low and is estimated to be less than 10%.
Polymerase chain reaction (PCR) carries a high degree of sensitivity and specificity for the detection of microorganisms in synovial fluid and tissue, even in individuals who are culture negative.15 Most bacteria can be detected on the basis of amplifying specific sequences in their ribosomal RNA (16S rRNA). PCR has been shown to be the procedure of choice for making the diagnosis of gonococcal arthritis16,17 and is a highly sensitive and specific method of detecting tuberculous arthritis, although as discussed later, analysis of synovial tissue is better than analysis of synovial fluid for making this diagnosis.18,19 PCR has also been proposed as a method of verifying the successful elimination of the offending organism in cases of septic arthritis.20,21
The sensitivity and specificity of PCR in detecting synovial microorganisms need to be balanced against the biologic significance of a positive test. Contaminants are easily detected using this method, and highly stringent conditions for sample collection are required to prevent false-positive tests. Moreover, PCR studies of synovial fluid and tissue from a spectrum of chronic forms of arthritis, including RA, osteoarthritis, reactive arthritis, and undifferentiated arthritis, have indicated the presence of microorganisms in a significant number of specimens.22,23 The biologic significance of these findings and the potential role of bacterial DNA or cell wall fragments in the pathogenesis of these arthropathies remain unclear.
Biochemical Analysis of Synovial Fluid
A number of widely available biochemical tests may add to the diagnostic impression of aspirated synovial fluid samples, although lack of specificity of these biochemical analyses tends to limit their value.12,24 Testing for synovial fluid glucose, protein, and lactate dehydrogenase (LDH) has long been included in routine practice, and values obtained should be compared with serum values. Samples from septic arthritis typically exhibit very low glucose, low pH, and high lactate levels; these levels are indicative of a switch to anaerobic metabolism. Highly inflammatory synovial fluids from RA patients exhibit a similar profile, along with high protein and LDH levels. Levels of pressure of oxygen in the blood (pO2) are often in the hypoxic range in RA synovial fluids, and are correlated with increased lactate and levels of pressure of carbon dioxide in the blood (pCO2).25,26 A prospective study conducted to evaluate these tests in a spectrum of inflammatory and noninflammatory disorders demonstrated considerable variability in each diagnostic category, which limits their clinical utility.24
Serologic testing of synovial fluid to detect rheumatoid factor, antinuclear antibodies, and complement levels has been suggested as a method that can be used to confirm a diagnosis of RA or other connective tissue diseases. In particular, RA synovial fluids may be positive for rheumatoid factor, even when serum is not,27 and complement levels are typically low as a result of consumption by immune complexes. These findings are of insufficient sensitivity and specificity to be of value on a routine clinical basis.
Synovial Fluid Analysis in Arthritis Research
The ease with which synovial fluid is aspirated from effused joints has allowed a wide spectrum of research studies to be conducted on this biologic material. In research settings, cells in synovial fluid samples are typically separated by centrifugation, and cellular and noncellular components of the fluid are analyzed separately. Detailed analysis of the phenotype and functional properties of synovial fluid leukocytes has been particularly informative in RA and reactive arthritis research, where immunophenotyping of lymphocyte subpopulations has provided important clues to the pathogenesis of these diseases. In the case of reactive arthritis, in which triggering organisms are often identified, the proliferative and cytokine responses of synovial fluid lymphocytes to antigens derived from Chlamydia, Yersinia, and other pathogens have been elucidated.28,29 It has generally been shown that synovial fluid T cells from reactive arthritis patients are biased toward production of T helper (Th)2 cytokines such as interleukin (IL)-10 and IL-4, whereas synovial fluid T cells from RA patients are Th1 biased and exhibit defects in Th2 differentiation.30–32
Analysis of the noncellular portion of synovial fluid has provided important information regarding a spectrum of soluble molecules, including cytokines and growth factors,33 extracellular matrix proteins, autoantibodies, and therapeutic drug levels. Moreover, broad-based proteomic studies of synovial fluid using fractionation techniques and mass spectrometry are beginning to provide novel approaches to understanding pathogenesis and prognosis in arthropathies such as RA.34
Synovial Biopsy
Blind Percutaneous Synovial Biopsy
Percutaneous needle biopsy is most commonly performed according to the method originally described by Parker and Pearson,35,36 utilizing a biopsy needle that now carries their name. Percutaneous synovial biopsy is most often performed on the knee joint, although the technique can readily be adapted for use in other joints such as wrist, elbow, ankle, or shoulder. A modification of the original Parker-Pearson needle has facilitated synovial biopsy of small hand joints such as metacarpophalangeal and proximal interphalangeal joints.37 The technique for Parker-Pearson synovial biopsy uses a 14-gauge needle with a lateral aperture just proximal to the inserted end of the needle. This lateral opening features a sharp cutting edge for severing trapped synovial tissue that is captured by applying suction with a 3- to 5-mL syringe. With this approach, multiple 1- to 3-mm samples are obtained by angling the trocar in several directions. This also serves to minimize the sampling error involved. Synovial samples are typically pink and are easily removed with a slight twisting motion. Because of the blind nature of the procedure, samples of fat, muscle, or fibrous tissue may be obtained and need to be separated from true synovial samples.
Percutaneous synovial biopsy is easily performed in most ambulatory care settings with the use of relatively inexpensive equipment. The overall morbidity of the procedure is low and is comparable with that of arthrocentesis, with perhaps a slightly higher rate of hemarthrosis, which can be minimized if the patient does not bear weight for a few hours after the procedure. The main disadvantage of the procedure is its blind nature. In comparison with visually guided arthroscopy, samples derived from the interface between synovium and adjacent cartilage are under-represented when the blind procedure is used.38,39 As is discussed later, this drawback is particularly relevant to a number of research questions.
Arthroscopically Guided Synovial Biopsy
The primary advantage of arthroscopy is its ability to visually guide the biopsy procedure. This permits macroscopic evaluation of the synovium and sampling of areas that appear to be particularly severely affected by the pathologic process, and it allows for sampling of the interface between inflamed synovium and adjacent cartilage, this being an area of particular interest for understanding the pathogenesis of destructive arthropathies such as RA.38 As with samples obtained by percutaneous synovial biopsy, individual samples are allocated for specific laboratory studies depending on the clinical or research question being addressed.
Processing Synovial Tissue Samples
Several antigen retrieval methods are available, including enzymatic and thermal methods,40 which have been used to successfully retrieve a spectrum of antigens from archival synovial tissue samples for immunohistologic studies, although the quality of the tissue sections often deteriorates after antigen retrieval. A number of double-staining immunohistology techniques have been developed for simultaneous evaluation of the expression of two markers in the same tissue section, although these techniques are labor intensive and often require considerable experimentation to generate good stains.41 It should be noted that formalin fixation dissolves crystals, and if this is a diagnostic consideration, the specimen should be fixed in ethanol.
The sensitivity and specificity of molecular DNA and RNA techniques provide unprecedented opportunities for exploring the pathogenesis of synovial disorders. Although these studies can be carried out on very small quantities of tissue, great care needs to be taken in handling and processing tissue samples to prevent degradation of the nucleic acids, particularly in the case of RNA when RNAase enzymes are ubiquitous and can rapidly degrade the small quantity of RNA present in a tissue sample. As is discussed later, the search for microbial DNA and RNA has been of particular interest in attempts to understand the cause and pathogenesis of reactive arthritis, rheumatoid arthritis, and other forms of chronic synovitis of unknown cause. Techniques used to analyze human gene expression in small tissue samples have been rapidly improved. This has enabled the detection and quantitation of multiple mRNA transcripts in very small quantities of biopsy material, in many cases without the need for amplification.42,43
Synovial Pathology
Synovial Membrane in Health
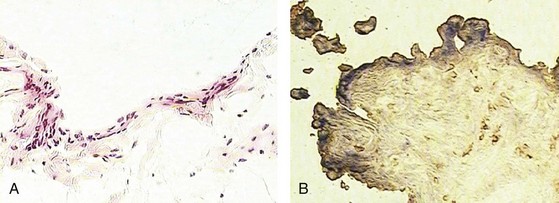
A detailed description of the composition of normal synovium is provided in Chapter 2. Histologically, the normal synovial lining layer is one to three cells thick and is composed of closely associated macrophage-like (type A) and fibroblast-like synoviocytes (type B) that are not separated from the underlying stroma by a basement membrane, as is the case with a true epithelium. In many areas, visible gaps in this lining layer allow small molecules to easily diffuse through the extracellular matrix into the synovial fluid. The two types of lining layer synoviocytes are distinct and can be differentiated on the basis of ultrastructural and immunohistologic features. Macrophage-like synoviocytes are myeloid in origin, as they exhibit the morphologic characteristics of phagocytic cells and express macrophage markers such as CD68, CD14, and FcγRIIIa. Fibroblast-like synoviocytes are synthetic cells of mesenchymal origin that are the primary source of hyaluronan and other proteoglycans found in normal synovial fluid. They express CD55 (decay-accelerating factor [DAF]), high levels of vascular cell adhesion molecule (VCAM)-1, and the enzyme uridine diphosphoglucose dehydrogenase (UDPGD), which is involved in the synthesis of hyaluronan and has been detected by cytochemical methods (Figure 53-6). Fibroblast-like synoviocytes have also been shown to uniquely express cadherin-11, a specialized adhesion molecule that is involved in homotypic aggregation of these cells and that contributes to maintaining the integrity of the synovial lining layer.44 Quantitatively, most of the cells in the normal synovial lining layer are synthetic type B cells. The underlying stroma features a rich network of capillaries with fenestrated endothelium in the immediate sublining area that serve to maintain the health and viability of adjacent cartilage. Larger arterioles and venules can be found deeper in the synovial stoma. The synovial microvasculature is surrounded by loose connective tissue, which also incorporates the synovial lymphatics that serve to drain this tissue. It has been shown that the synovium of completely asymptomatic individuals not uncommonly exhibits a modest infiltrate of T lymphocytes that are occasionally organized in perivascular aggregates, although B cells were not seen.45
Synovial Histopathology in the Evaluation of Monoarthritis
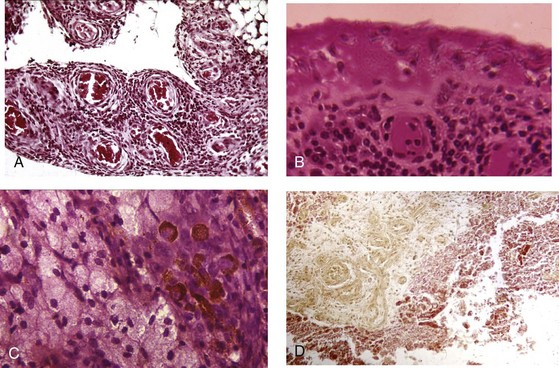
Pathologic analysis of synovial tissue samples can be of considerable value in certain clinical settings. Having said this, it should be kept in mind that the histopathologic interpretation of synovial biopsy specimens is often nondiagnostic and lacking in specificity.46 Pathologic analysis of synovial samples from patients with undiagnosed monoarthritis may be of particular value. The presence of large numbers of neutrophils in the synovial tissue stroma is highly suggestive of septic arthritis, and in such cases Gram stain may reveal bacteria in the tissue. Because septic arthritis is usually acute in onset, synovial biopsy is rarely required, and the diagnosis can be made by analyzing synovial fluid as described previously. Gonococcal arthritis may require synovial biopsy for diagnosis (Figure 53-7). A mononuclear cell infiltrate, on the other hand, is more consistent with a chronic inflammatory process and has a wide differential diagnosis, as has been described. The presence of granulomas supports a diagnosis of tuberculous arthritis or sarcoidosis, both of which cause chronic monoarthritis. The synovial granulomas of tuberculosis (TB) may be caseating or noncaseating, and staining of the tissue for acid-fast bacilli, culture, and molecular probing can yield a definitive diagnosis in an estimated 50% of cases. Similarly, a spectrum of fungal infections can be diagnosed using similar approaches, but special stains such as Gomori may be required. The diagnosis of sarcoid arthropathy is suspected in synovial specimens with noncaseating granulomas in cases where mycobacterial or fungal infection has been excluded.
Pigmented villonodular synovitis is an important consideration in individuals with chronic monoarthritis of a large joint such as the knee or hip. This disorder has a characteristic magnetic resonance imaging (MRI) appearance caused by hemosiderin deposits in the synovium and large cystic lesions in adjacent bone. Histopathologic analysis of the synovium can confirm this diagnosis and demonstrates a diffusely hypervascular proliferative lesion with mononuclear cells of the monocyte/macrophage lineage, foamy multinucleated cells resembling osteoclasts, and hemosiderin deposits47 (see Figure 53-7). Synovial sarcomas are rare tumors that must be diagnosed on the basis of synovial pathology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree