Bone marrow replacing disease processes, such as Gaucher’s disease [5]
Dysbaric disorders, such as decompression sickness (Caisson’s disease, aka the “bends”)
Systemic lupus erythematosus [6]
Inflammatory bowel disease
History of undergoing organ or tissue transplant [7]
Chronic renal failure
Pancreatitis
Pregnancy
Pathophysiology
The pathophysiology is poorly understood, making it difficult to prevent this condition. The pathogenesis can be multifactorial, with a combination of metabolic factors and local/host factors affecting blood supply to the femoral head. The disease process is thought to involve an interruption to the vascular supply of the femoral head, which causes adjacent hyperemia, demineralization of the bone, and trabecular thinning, leading to subchondral collapse. Specifically, coagulation of intraosseous microcirculation occurs, followed by resultant venous thrombosis and retrograde arterial occlusion, which increases intraosseous pressure, leading to decreased blood supply to the femoral head. This ultimately leads to death of osteocytes and osteoprogenitor cells, resulting in subchondral fracture and collapse. In cases with underlying traumatic etiologies, typically injury to the medial femoral circumflex artery is responsible. As such, osteonecrosis is most common in cases of displaced femoral head fractures, followed by basicervical femoral neck fractures; the highest risk being combined intrafoveal femoral head and displaced femoral neck fractures (Pipkin III). Hip dislocations are also associated with the development of osteonecrosis; however, if the joint is reduced within 6–8 h of injury, the rate of osteonecrosis is decreased [11, 12].
Clinical Presentation
History and Physical Examination
Patients with femoral head osteonecrosis can be asymptomatic and the diagnosis can be made from imaging performed for another indication. The vast majority of symptomatic patients complain of progressive pain localized to the groin and general hip region. While pain localized to the groin is most specific for intra-articular pathology, pain in the thigh and buttock is also commonly seen. These symptoms can be exacerbated with weight bearing and with combined flexion and internal rotation. Patients should be asked about prior history of trauma, surgery, corticosteroid use, ethanol use, and personal or family history of blood disorders. Sudden inability to bear weight associated with fevers, chills, weight loss, and/or night sweats warrant further workup for infectious, inflammatory, and or malignant underlying etiologies. Physical examination findings can often be nonspecific. Patients may walk with a mild antalgic gait favoring the affected side, but otherwise the majority of examination findings are normal during early stages of the condition. Any neurovascular deficits or isolated muscular weakness should be further worked up for other underlying etiologies.
Imaging Studies
All patients with suspected osteonecrosis of the femoral head should undergo a complete radiographic workup including an anterior–posterior (AP) view of the pelvis, an AP view of the affected hip, and a cross-table or frog-leg lateral view of the affected hip. Often, it is the lateral view that best demonstrates subchondral collapse and/or collapse. The most commonly utilized classification systems for osteonecrosis are based on radiographic findings and historically include the Ficat classification [13], and more recently the Steinberg classification (Table 2) [14]. Of note, patients who are diagnosed with AVN in another area of the body (i.e., humeral head) should always get hip radiographs as there is an increased risk of having concomitant (but asymptomatic) femoral head AVN [15]. Radiographs can remain completely normal for months after symptoms begin (Fig. 1). Early radiographic findings of osteonecrosis will show mild density changes within the femoral heads due to micro-infarcts with corresponding calcification. In both of these systems, stage III will show a crescent sign radiographically, indicating subchondral collapse (Fig. 2). Typically, advanced imaging with magnetic resonance imaging (MRI) is also employed if there is a high index of suspicion and/or the patient is at risk (i.e., alcoholic) despite normal radiographs. This modality can reveal changes due to osteonecrosis at earlier stages than plain radiographs are capable of showing. MRI has been shown to have excellent specificity and sensitivity with regard to osteonecrosis and will typically appear bright on T2-weighted images with corresponding dark findings on T1-weighted images. Bone scan is also another modality that can be used to help diagnose and stage osteonecrosis of the femoral head, but is not as specific as MRI [16, 17].
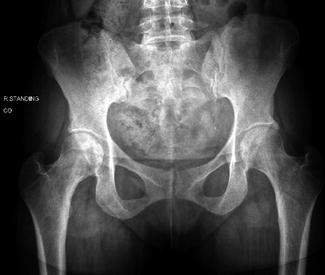
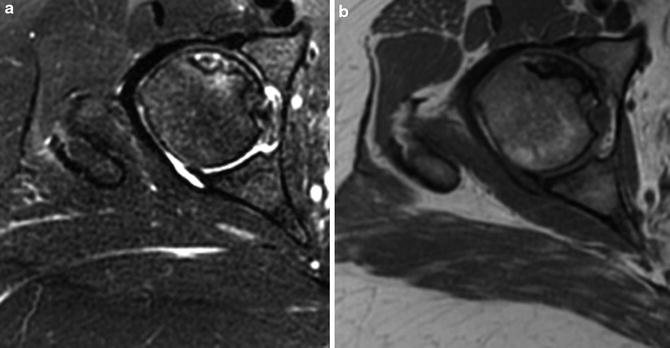
Table 2
Steinberg classification system
0 | Normal XR, normal MR |
I | Normal XR, abnormal MR (and/or bone scan) |
II | Cystic/sclerosis, abnormal MR (and/or bone scan) |
III | Crescent sign (subchondral collapse), abnormal MR (and/or bone scan) |
IV | Femoral head flattening, abnormal MR (and/or bone scan) |
V | Narrowing of joint, abnormal MR (and/or bone scan) |
VI | Advanced degenerative changes, abnormal MR (and/or bone scan) |

Fig. 1
AP radiograph of the pelvis demonstrating well-preserved joint space without evidence of collapse, cam, pincer, or dysplasia of the bilateral hips

Fig. 2
Axial cuts from MRI demonstrating osteonecrosis (ON) of the right anterior superior femoral head (a: T2-weighted image, b: T1-weighted image)
Management Options
Based on symptoms and imaging findings, osteonecrosis of the femoral head is typically divided into early, or pre-collapse, versus late, or post-collapse, stages. Treatment options vary depending on the stage, with late stage presentations the most difficult to manage (from a non-arthroplasty perspective). Without any intervention, the natural history of osteonecrosis is that of ultimate disease progression, with most patients ultimately progressing to femoral head collapse and end-stage arthritis [18, 19]. Asymptomatic patients typically present in the pre-collapse stages and can be managed nonoperatively with close clinical follow-up. These patients must be watched closely, and the development of pain should prompt a repeat examination with repeat imaging studies.
Patients who are symptomatic and in the pre-collapse stages will most often require intervention. While some medical therapies including marrow/stem cells [20–24] and bisphosphonate [25] use have been advocated, current results are inconsistent, and the majority of patients will undergo surgical intervention. Surgical options for these pre-collapse patients are outlined in Table 3. Non-arthroplasty options include core decompression , vascularized bone graft [26–30], non-vascularized bone graft, and osteotomies. Treatment options for post-collapse patients, however, are more limited, given the advanced stage of the disease. While most post-collapse osteonecrosis patients can be successfully managed with total hip arthroplasty [31], joint replacement may not be the best option in young, active patients, given the obvious concern for early polyethylene wear with subsequent osteolysis, component loosening, osteolysis, and the potential need for early revision.
Table 3
Surgical options for osteonecrosis of the femoral head
Core decompression |
Rotational osteotomy |
Vascularized graft transfer (free fibula) |
Non-vascularized graft transfer |
Total hip arthroplasty |
Unipolar or bipolar hemiarthroplasty |
Hip resurfacing |
Core decompression is a surgical technique in which the necrotic lesion is reamed or drilled to decrease local intraosseous pressure and stimulate a vascularized healing response. Multiple authors have demonstrated effective results with this treatment strategy for pre-collapse osteonecrosis, including arthroscopic-assisted decompression [32–34]. Given recent advances in techniques and instrumentation, hip arthroscopy is now the gold standard for the diagnosis of intra-articular hip pathology [35, 36]. Substantial improvements in hip-specific diagnostic modalities have improved the understanding of bony and soft tissue pathology in patients with intra-articular hip disorders. The association of concomitant soft tissue and/or bony pathology with osteonecrosis of the femoral head is currently unknown. Further, the ability of imaging studies to predict collapse and/or associated hip pathology in addition to osteonecrosis at the time of surgery is also unknown. Thus, arthroscopy at the time of decompression provides an accurate means to confirm the presence or absence of femoral head subchondral collapse, chondral delamination, and associated labral, capsular, and synovial pathology [37–39]. If present, such concomitant pathology can be addressed at the time of decompression and obviate the need for a subsequent surgery. Further, arthroscopy allows for verification and guidance during drilling and/or reaming to avoid penetration of the articular surface. The following section describes the surgical technique [40] for arthroscopic-assisted core decompression of the femoral head (Table 4).
Table 4
Surgical steps
Establish anterolateral and anterior portals |
Evaluate femoral head for subchondral collapse |
Evaluate integrity of articular cartilage |
Evaluate labrum and bony margin of acetabulum |
Interportal or T-capsulotomy to address concomitant labral and/or femoral head–neck pathology |
Maintain arthroscope in the joint to assist in avoiding subchondral penetration |
Introduce drill and reamer under X-ray guidance, lesion typically in anterior superior femoral head |
Introduce arthroscope into socket to confirm integrity of subchondral bone at lesion site |
Implant ceramic putty to provide compressive strength |
Perform dynamic arthroscopy of femoral head |
Arthroscopic-Assisted Core Decompression: Surgical Technique
Patient Positioning and Surgical Setup
The patient is positioned supine on a traction table with a well-padded perineal post placed in the groin between the legs (Smith and Nephew hip traction system, Smith and Nephew, Andover, MA). Traction is applied with the leg in neutral extension, axially distraction, and adduction to provide a cantilever moment to the operative hip.
Landmarks
The greater trochanter and anterior superior iliac spine borders are palpable landmarks used to identify appropriate portal placement. These are marked on the skin.
Portals
Under fluoroscopic visualization, a standard anterolateral (AL) portal is created 1 cm proximal and 1 cm anterior to the AL aspect of the greater trochanter. The 70° arthroscope is inserted over a guidewire. While viewing from the AL portal, needle localization is used to establish an anterior portal, penetrating the capsule at the 2 o’clock position. The anterior portal is approximately 1 cm lateral to the line drawn vertically from the anterior superior iliac spine (ASIS) and a line drawn horizontally from the AL portal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








