Fig. 1
Specimen from an 18-month-old human cadaver. Evident is the complete independence of the lateral epiphyseal arteries investing the epiphysis and the ascending metaphyseal vessels ending at the physeal plate. This independence persists until physeal closure in adolescence (From Trueta, J: The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br 1957;39-B(2):358–394, with permission)
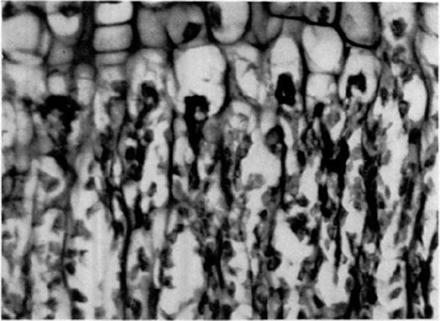
Fig. 2
During adolescence, terminal branches of the ascending metaphyseal circulation do not cross the closing physis, instead ending in the hypertrophic zone of the growth plate (From Trueta J: The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br 1957;39-B(2): 358–394, with permission)
Preceding the above experiments, Trueta described the intra- and extraosseous proximal femoral vascular anatomy in adult hips through a series of detailed histological dye studies [9]. The medial circumflex femoral artery (MFCA) branches from the profunda femoris artery, has a predictable branching pattern, and supplies lateral epiphyseal branches which become the dominant vascular contribution to the femoral head by age of 18 months. This dominance persists into adulthood. The MFCA ascends the posterolateral femoral neck and, once intracapsular, is invested by a fibrous sheath and adjacent venular system. The artery generally divides into 2–6 spiral branches and heads toward a point between fovea and the inferior articular margin (Fig. 3). These arteries undergo a characteristic arborization in both the coronal and sagittal planes, almost always branching at 90° and directed toward the chondral surface, providing a robust subchondral circulation (Fig. 4). In adulthood, little ascending metaphyseal contribution exists to the femoral head, and only a small anastomosis with the artery of the ligamentum teres (the acetabular branch of the obturator artery) may remain. The artery of the ligamentum teres was found in 100 % of cadaveric specimens aged 13–80 years in a study by Wertheimer et al. [10], but more than two third did not fill with dye on histological section, could not be visualized angiographically, or were of such small caliber to be deemed clinically insignificant. In 18 % of specimens, an anastomosis of the lateral epiphyseal with the ascending metaphyseal arteries was present and was more common in females. Such an anatomical variant is thought to be protective and to explain the lower rate of avascular necrosis (AVN) in female patients with SCFE [11]. Ganz and colleagues performed detailed dye investigations of the MFCA and lateral epiphyseal vessels, the protection of which can allow for complex surgical exposure of the hip by preservation of the epiphyseal circulation [12].
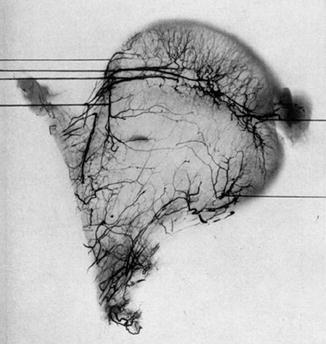
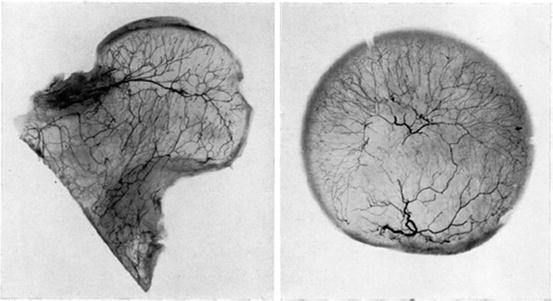

Fig. 3
The medial circumflex femoral artery becomes intracapsular in the posterolateral femoral neck, and its lateral epiphyseal vessels provide the majority of the nutrition to the femoral head after physeal closure. There is minimal ascending metaphyseal contribution and varying but small anastomoses with the artery of the ligamentum teres (From Trueta J and Harrison MH: The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br 1953;35-B(3): 442–461, with permission)

Fig. 4
The intraosseous lateral epiphyseal vessels undergo characteristic branching at right angles in all planes and point toward the chondro-osseous junction (From Trueta J and Harrison MH: The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br 1953;35-B(3): 442–461, with permission)
Osseous and Physeal Anatomy
At first consideration, a slipped capital femoral epiphysis may appear similar to a Salter-Harris I growth plate injury. However, multiple factors differentiate SCFE from an acute fracture, including antecedent physeal lysis, slower displacement, and intact periosteum. There are a number of anatomical characteristics that likely contribute to the pathogenesis of SCFE and physeal instability, including variations in proximal femoral and acetabular anatomy.
Gelberman et al. [13] compared torsional profiles from axial CT scans of femora with unilateral slipped epiphyses and described relative femoral retroversion in affected hips (averaging 1.0 vs. 6.3° of anteversion). He postulated that abnormal torsional stresses, stemming from decreased anteversion, could contribute to rotational instability across the developing growth plate. Later, Sankar investigated acetabular anatomy after treatment of unilateral SCFE, demonstrating acetabular retroversion and over-coverage in the unaffected hip, a finding with implications for SCFE development and the development of posttreatment impingement in the unaffected hip [14].
The femoral neck-shaft angle decreases from 160° at birth to an average of 125º by adolescence with changes in orientation of the physis. Speer et al. described a growth spurt around age 7 resulting in asymmetric neck lengthening and increased verticality of the physis [15]. Mirkopoulos et al. [16] found that patients with unilateral SCFE had steeper physes than both their unaffected contralateral hips and age-matched controls. A 14° increase in radiographic slope occurred between 1 and 18 years, with the most rapid increase occurring between ages 9 and 12 with no relationship to sex or other demographic factor. This higher “physeal inclination angle” and resultant increase in shear vector parallel to the growth plate may contribute to epiphyseal translation and development of an SCFE [15, 17]. Since physiologic loads can create shear forces in excess of six times body weight, obesity further contributes to epiphyseal instability.
The perichondrial ring also contributes to the load-carrying capacity of the physeal plate. The perichondrial ring thins and attenuates during development, and consequently less shear force is necessary to cause epiphyseal displacement by early adolescence [15, 18]. Physiologic shear forces alone can displace an adolescent proximal femoral epiphysis ex vivo [18, 19], furthering the mechanical contribution to SCFE development.
Although the exact pathogenesis is unclear, physeal columnar height and organization are significantly altered in SCFE. Because of the already thinned perichondrial ring after 3 years of age, the large surface area of the undulating, interlocking mammillary processes provides the greatest internal support of the epiphysis. SCFE is characterized by physeal widening up to 12 mm (normal range, 2–6 mm), a widened hypertrophic zone comprising 60–80 % of the physeal height, enlargement of chondrocytes, columnar disorganization, cleft formation, higher proteoglycan and extracellular matrix concentrations throughout the physis, and a general disruption in orderly chondrocyte differentiation and endochondral ossification (Fig. 5) [15, 17, 20]. Radiographic physeal widening implies a mechanically weakened physis susceptible to “unlocking” of the mammillary processes and further destabilization.
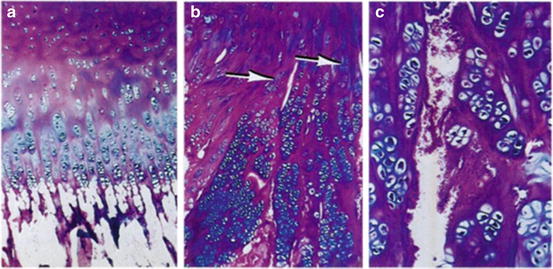

Fig. 5
Normal proximal femoral physis with extracellular matrix primarily in the resting zone and excellent columnar organization of developing chondrocytes (a). Proximal femoral physis of an SCFE patient showing ECM in the proliferating and hypertrophic zones (b) and a frank cleft in the hypertrophic zone with disorganized ECM and erythrocyte invasion (c) (From Ippolito E, Mickelson MR, Ponseti IV: A histochemical study of slipped capital femoral epiphysis. J Bone Joint Surg Am 1981;63(7):1109–13, with permission)
The epiphyseal tubercle is an anatomical feature receiving increased attention. It is a prominence consistently located among the mammillary processes of the posterosuperior quadrant of the epiphysis [21]. The tubercle averages 4 mm in height, is always below the foraminae for the lateral epiphyseal vessels, and is postulated to confer mechanical strength to the physeal plate. It is considered a possible “keystone” for physeal stability, decreasing in normative size and surface area during childhood and adolescence with a concomitant increase in peripheral physeal cupping, which is thought to provide compensatory stability. Liu et al. postulate that the epiphysis internally rotates on the epiphyseal tubercle and that a widened physis could contribute to epiphyseal dislodgement [22]. Since the lateral epiphyseal arteries are immediately adjacent to and above the epiphyseal tubercle, this could explain the low rate of osteonecrosis in chronic, stable slips (i.e., minimal displacement of the lateral epiphyseal vessels; Fig. 6).
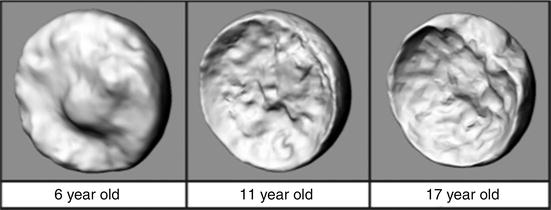

Fig. 6
Three-dimensional CT reconstructions of the epiphyseal tubercle in the posterosuperior epiphysis, decreasing in normative size as a child ages. Also appreciable is the increase in physeal cupping over time (From Liu RW, Armstrong DG, Levine AD et al.: An Anatomic Study of the Epiphyseal Tubercle and Its Importance in the Pathogenesis of Slipped Capital Femoral Epiphysis. J Bone Joint Surg Am 2013;95:e34(1–8), with permission)
Related Conditions
Suspicion of an endocrinologic disturbance in the pathogenesis of SCFE arose due to the known stippling effect of congenital hypothyroidism, as thyroid hormone (T3) is necessary for normal skeletal development and specifically chondrocyte differentiation. While a common presentation of SCFE is that of an obese, “hypogonadal” male during the adolescent growth spurt, the majority of SCFEs occur in the absence of endocrinopathy [23, 24].
A stature test has been used to identify patients with SCFE and a concomitant endocrine abnormality [25]. Patients with SCFE who were below the 10th percentile for height comprised 90.9 % of all endocrinopathies, and all such patients remained below the 10th percentile throughout adolescence. These children could be identified with a sensitivity of 90 % and negative predictive value (NPV) of 98.6 % based on height alone and hence could be targeted with screening laboratory tests and appropriate workup. Loder et al. described and later reaffirmed the prognostic implications of an “age-weight” and “age-height” test to distinguish a typical (idiopathic) from an atypical (usually endocrine-related) SCFE [26]. Patients were grouped into six categories based on age (<10 years, 10–16 years, >16 years) and weight or height (greater or less than the 50th percentile). In combined variable models, extremes of age and weight; height and weight; and age, height, and weight were associated with increased odds ratios of an atypical slipped epiphysis (Table 1). As these tests all have high NPV, an orthopedic surgeon can feel reasonably confident that an SCFE is idiopathic when the age-weight, age-height, and stature tests are negative. Laboratory screening will not be cost-effective unless it is guided by specific patient characteristics and appropriate clinical suspicion.
Table 1
Odds ratios of atypical SCFE based on deviations from norms of age, weight, and height (Adapted from Loder RT, Starnes T, Dikos G: Atypical and typical (idiopathic) slipped capital femoral epiphysis. Reconfirmation of the age-weight test and description of the height and age-height tests. J Bone Joint Surg Am 2006;88(7):1574–81, with permission)
Group | Odds ratio |
|---|---|
Age <10 or >16 years | 7.4 |
Height <50th percentile | 6.0 |
Age and height | |
Age <10 or >16 years | 5.8 |
Height <50th percentile | 15.0 |
Weight and height | |
Weight <50th percentile | 7.4 |
Height <50th percentile | 12.8 |
Age, weight, and height | |
Age <10 or >16 years | 4.9 |
Weight <50th percentile | 4.5 |
Height <50th percentile | 14.1 |
Loder et al. [27] published a review of all previously reported slipped capital epiphyses in patients with endocrine disturbances, separating patients into three groups: hypothyroidism, growth hormone deficiency, and all others. Hypothyroidism was the most common diagnosis (40 % of 85 patients), and in his series, the development of SCFE usually antedated the diagnosis of thyroid disturbance that was generally made during the same hospitalization. All patients with growth hormone deficiency (25 %) that had been diagnosed prior to developing an SCFE experienced the shortest symptom duration before a slip diagnosis, and 92 % developed a slip during their hormone replacement therapy. All patients with other endocrine disturbances such as panhypopituitarism, craniopharyngioma, and multiple endocrine neoplasia (35 %) were diagnosed later in adolescence (average age 17.4 years) and an average of 3 years from first SCFE symptoms. Bilaterality has been reported more commonly in endocrine-related SCFE [27, 28], with many unilateral presentations progressing to contralateral involvement within the first 18 months. Increased rates of SCFE have also been demonstrated in patients with renal osteodystrophy and secondary hyperparathyroidism [29], postradiation [30], hypogonadism [31], and Down syndrome [32].
A unique combination of histological, vascular, anatomical, and endocrinologic factors all contribute to physeal stability and the pathogenesis of SCFE. The proximal femoral physis represents an area of rapid cellular proliferation more vulnerable to instability than areas of slower turnover, is characterized by a unique temporal susceptibility that can be heightened by the body’s endocrinologic milieu, and is nourished by a fragile blood supply. A thorough understanding of the complexity of proximal femoral anatomy is necessary before any surgical treatment of SCFE is undertaken.
Clinical Evaluation
Patients with slipped capital epiphyses can present to a physician in a delayed or acute fashion, often providing clues to chronicity and stability. Patients may have long-standing symptoms lasting months prior to physician evaluation. The most common presentations include limp and pain in the affected groin, lateral or posterior hip, thigh, or ipsilateral knee. Knee pain in SCFE is referred, which implies a reflex arc involving somatic sensory nerves ending at the same spinal level (Fig. 7). This differs from radiating pain, which is thought to be caused by irritation of obturator nerve branches that course to the medial knee. Knee pain, present in 15–50 % of SCFE, leads to high rates of misdiagnosis, extra radiographs, and higher-grade SCFE at treatment [33, 34] and could result in errant surgical procedures directed at nonexistent knee pathology or a second knee diagnosis such as Osgood-Schlatter [35], reinforcing the need for careful evaluation. A stable slip, Medicaid insurance, and distal thigh/knee pain are the strongest independent predictors of a delay in diagnosis of SCFE [34].
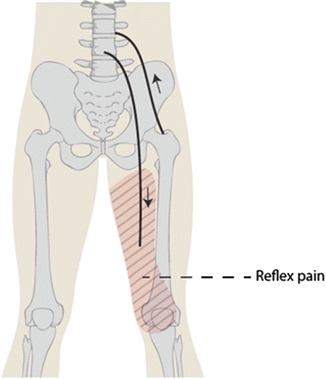

Fig. 7
Reflex arc of referred pain in SCFE, in which a reflex of afferent somatic sensory nerves from the hip terminates at a spinal level in proximity to efferent pain signals to the knee and thigh
Altered gait patterns in SCFE include components of antalgic, “waddling,” or Trendelenburg gait, with an externally rotated foot progression angle. Only a small percentage of limp in SCFE is painless [36]. Motion can be severely restricted in multiple planes, and rotational profiles should be compared bilaterally. Patients may have weak hip abduction, decreased hip flexion, decreased internal rotation, obligate external rotation with hip flexion (Drehmann sign), and the development of synovitis precipitating a flexed posture or even hip flexion contracture. A positive Drehmann sign is elicited when the examiner passive flexes the supine patient’s hip, which then falls into obligate external rotation and abduction [37].
Reviews of large national databases report an SCFE incidence rate of 10 per 100,000, with a 1.4:2.0 female/male ratio. The average age of diagnosis is 12 years, and most patients presenting outside of ages 10 and 16 represent atypical SCFE that may be associated with endocrinologic diagnoses [26]. Blacks, Hispanics, and Pacific Islanders have significantly greater incidence rates than Whites. There has been a consistent finding of higher SCFE incidence north of 40° latitude, with the greatest incidences in the United States found in the Northeast and West and the lowest rates in the Midwest and South. A seasonal incidence pattern has also been noted, with most northern latitudes presenting in the summer months and most southern latitudes in the winter [38, 39]. Recent data have indicated a trend of decreasing age and increasing frequency of bilaterality at first presentation, with a suspected correlation with increasing rates of childhood obesity [40].
Bilateral SCFE at first presentation has traditionally been reported in approximately 20 %, with higher rates in patients with endocrinopathy [25, 26]. The incidence of metachronous slip, affecting 15–36 % of SCFE, may provide justification for prophylactic pinning of the unaffected hip in at-risk patients [41, 42]. Almost 90 % of metachronous SCFE presents within the first 18 months after treatment of the index slip [43].
Radiographic Evaluation
The primary radiographic test used in suspected SCFE remains an AP and frog pelvis radiograph. Klein et al. [44] described a line constructed along the superior neck on the AP image that should intersect the epiphysis. Failure to do so comprises a positive “Trethowan sign” and may indicate a slipped femoral epiphysis. The sensitivity of this line has been questioned, reportedly missing 61 % of SCFE and underdiagnosing patients in a “pre-slip” phase [45]. Green et al. found similarly low sensitivity of this line, proposing a modification wherein the amount of the epiphysis lateral to Klein’s line was compared between the two hips on the AP image, with a 2 mm side-to-side difference highly suspicious of SCFE [46]. There are numerous subtle radiographic findings indicative of early slippage, including widening and irregularity of the affected physis, sharpening of the metaphyseal border of the head, loss of anterior concavity to the head-neck junction on the lateral view, and subtle periosteal elevation [44, 47]; however, lateral radiographs are generally more sensitive than the AP images, particularly in the earliest phases of slipping (Fig. 8). Most SCFE is characterized by anterior translation and external rotation of the metaphysis relative to the epiphysis, which, on an AP projection, is located posterior and inferior to the metaphysis. As a result, the total epiphyseal height may appear decreased and a double-density or “metaphyseal blanch” sign described by Steel [48] can be present. Chronic SCFE can result in uncoverage and resorption of the superior metaphysis, with periosteal reaction, osseous formation, and beaking along the inferomedial neck (Fig. 9).
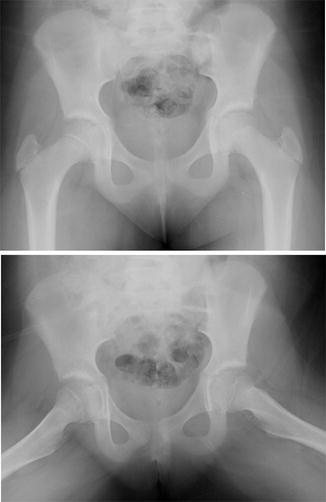
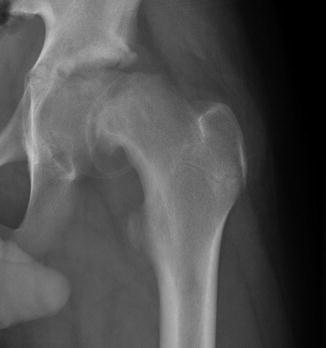

Fig. 8
AP and lateral radiographs of a right slipped capital epiphysis in a 10-year-old female. On the AP projection, Klein’s line intersects both epiphyses. A modified Klein’s line would reveal asymmetry in the quantity of epiphysis intersected. The right physis is obviously widened compared to the unaffected left. A frog-lateral projection reveals posterior slippage and loss of anterior convexity of the head-neck junction courtesy of Ira Zaltz, M.D.

Fig. 9
AP hip radiographic demonstrating chronic remodeling in chronic SCFE. There are periosteal reaction and beaking along the inferior neck and rounding and blunting of the superior metaphysis due to resorption courtesy of Ira Zaltz, M.D.
The role of advanced imaging is controversial and generally used on a case-by-case basis. Ultrasound can detect the presence of hip effusion and a step-off at the epiphyseal-metaphyseal junction [49], but provides little of the morphological detail necessary for surgical planning. Computed tomography (CT) allows for the assessment of physeal closure or morphological assessment of proximal femoral deformity, particularly with three-dimensional reconstruction if complex osteotomy is planned. Intra-articular hardware penetration is also best assessed by CT [50]. Magnetic resonance imaging (MRI) can assess vascularity to the femoral head or the degree of AVN, although hardware scatter can compromise image quality.
Classification
Symptom duration has been used to describe SCFE as acute, chronic, or acute on chronic. Acute SCFE comprises 10–15 % of all SCFEs and presents within 3 weeks of the onset of symptoms. Acute presentation is associated with a higher rate of AVN [51] and can present after trauma or an identifiable inciting injury. Chronic SCFE, comprising 85 % of cases, implies at least 3 weeks of symptoms, often presenting after prior physician evaluation or incorrect diagnoses, when symptoms fail to resolve. Patients with prodromal symptoms of pain and limp have been known to experience a new injury with immediate worsening, a so-called acute-on-chronic slip [11]. Temporal classifications are useful in describing chronicity but have less prognostic value.
Loder introduced the concept of physeal stability in his classification, categorizing SCFE as either “stable” or “unstable” based on the patient’s ability to bear weight, even with crutches [43]. Physeal stability was predictive of osteonecrosis rates, with 47 % of unstable and 0 % of stable slips developing AVN within 6–18 months. There is marked heterogeneity in the application of the stable/unstable classification in the literature [52]. More recently analyzed historical data suggests the rate of osteonecrosis in unstable SCFE is closer to 23.9 % [53].
Radiographic severity was originally described by epiphyseal displacement as a fraction of total physeal diameter, expressed as a percentage [47, 54]. By this grading, slips can be mild (<33 %), moderate (33–50 %), or severe (>50 %). A more commonly used classification is the slip angle of Southwick [55], in which the difference in the angle subtended by the proximal femoral physis and the ipsilateral femoral shaft is compared between affected and unaffected sides. Differences less than 30° are mild, between 30° and 50° are moderate, and greater than 50° are severe. Since femoral rotation can vary depending on radiographic technique and patient comfort, the Southwick grading is inconsistent. Cross-table and frog-lateral projections have been found to optimally quantify the magnitude of SCFE by the Southwick angle when the femur is abducted to 45° with less than 30° of external rotation [56]. In cases where a contralateral comparison is unavailable (i.e., contralateral slip), Southwick described “normal” head-shaft angles for ready comparison.
Treatment
Slipped capital femoral epiphysis demands surgical management except in rare instances, as stabilization of the epiphysis and early fusion of the proximal femoral physis prevents further displacement and is a common goal of all treatment modalities. The preferred treatment of SCFE has undergone dramatic change as surgical techniques have become more refined, and improved imaging has allowed complex definition of deformity and precise surgical planning. There remains considerable debate about the optimal treatment of stable SCFE. Recommendations will evolve if long-term, prospective data becomes available and as surgeons are trained in increasingly complex hip surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








