7 Regenerative Medicine and Tissue Engineering
With dramatic advances in targeted treatments for arthritic disease, inflammation can be kept under control quite efficiently. In addition, a better understanding of the destructive processes beyond inflammation in arthritic disease has identified new molecular targets, such as metalloproteinases, and cellular targets, such as the osteoclasts. These findings in turn have led to the development of new treatment approaches that effectively contribute to improved control of the destruction of joint and joint-associated tissues. The new challenge in arthritic disease is to position all these therapeutic opportunities properly, ultimately leading to a stratified approach (i.e., giving optimal treatment to the proper patient at the right time). New principles have emerged in the management of arthritic disease, such as early detection, remission induction and maintenance therapy, tight disease control, patients at risk and responders to treatment; these have become a part of daily clinical practice.1–3
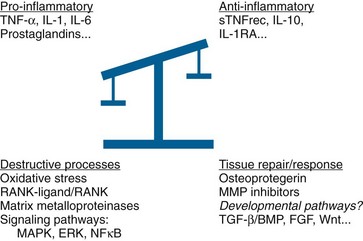
These developments and in particular the widespread use of new biologics such as tumor necrosis factor (TNF) inhibitors have highlighted the need to consider other aspects of joint biology, in particular the mechanisms driving tissue response and repair.4 Indeed, to restore the balance between tissue destruction and tissue repair (Figure 7-1), we should be looking arguably at the overview picture (i.e., the “systems biology” of the joint). Introducing regenerative medicine provides a novel opportunity to restore joint homeostasis, thus possibly leading to cure. Targeting repair has entered our discipline, and investigating the potential to activate and enhance joint tissue repair mechanisms has become a prime goal.
As an example of, and of relevance for, skeletal tissues, it appears that the process of rebuilding an adult limb has many similarities with how the limb is formed a priori in the embryo; signaling mechanisms are required to specify the final pattern. Thus limb formation and limb regeneration are likely to employ similar molecular pathways.5 Remarkable advances in developmental biology over past decades have provided the knowledge platform to advance into novel regenerative medicine approaches in postnatal life. These advances include not only improved understanding of the mechanisms of body axis formation and organogenesis, but also impressive progress in stem cell biology, including the regulation of stemness and stem cell niches, lineage specification and cell differentiation, and the critical molecular pathways involved.
In view of this, we are now in a position to enter a new era in regenerative medicine and tissue engineering.6,7 In this chapter, we will briefly review approaches seeking to repair damaged and diseased tissues, in particular diarthrodial joints and skeletal structures.
Intrinsic Tissue Repair
As the body strives to maintain homeostasis of postnatal tissues and thus undergoes continuous tissue remodeling, many signals counteract destructive processes in tissues. Disease processes may allow destructive processes to become dominant, leading to loss of tissue homeostasis and loss of function. Enhancement of expression/secretion of signals counteracting the breakdown processes is a straightforward approach to restore homeostasis. Naturally occurring examples include production of soluble receptors or receptor antagonists (sTNF receptors, interleukin [IL]-1RA), use of inhibitors of matrix modeling enzymes such as tissue inhibitors of metalloproteinases (TIMPs), and inhibition of osteoclast formation caused by blocking the receptor activator of nuclear factor κB (NFκB) (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) system. Some of these targets have reached the clinic; others are in early or late phase clinical trials across a range of diseases, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and osteoarthritis (OA). Note, however, that in some arthritic diseases such as ankylosing spondylitis, the tissue response is abnormal, leading to joint ankylosis (for review, see Lories et al8).
TGF-β/BMP Signaling
Recent reviews have highlighted the relevance and critical role of members of the TGF-β superfamily (TGF-β, BMPs, GDFs) in the biology of articular cartilage, bone, joints, and joint-associated tissues, during development, in postnatal tissue homeostasis and repair, and in tissue response on injury and aging. TGF-β has been shown to be involved in the maintenance and aging of articular cartilage and in osteoarthritis.9 BMPs have been reported to play major roles in articular cartilage metabolism, with BMP7/OP1 being of particular interest.10,11 In addition, data indicate that modulation of TGF-β/BMP downstream receptor-Smad signaling plays an essential role in both the regulation of chondrocyte differentiation and the development and progression of osteoarthritis.12 It is therefore not surprising that because of overwhelming evidence of the regenerative potential of this family of growth and differentiation factors in preclinical models, their therapeutic potential is being explored. In particular, several ongoing phase I studies on osteoarthritis of the knee are seeking to target OP1/BMP7 (clinicaltrials.gov). In addition, a search is ongoing, through peptide technology (peptidomimetics) or small molecule screens, to identify modulators of the TGF-β/BMP receptor/Smad signaling pathways.13 Because the nature of the synovial joint allows for local treatment, it is expected that some of the newly identified compounds will first be tested in local application such as joint surface repair. Indeed because this family of proteins, in particular the BMPs, plays a role in many systemic processes, BMP technology may be required to focus in the first place on local treatment, as exemplified in the use of BMP devices already in the clinic for orthopedic applications such as spine fusion and healing of nonunion.14,15 Wider systemic morbidity upon targeting BMP pathways is as yet unclear.
FGF Signaling
Extensive investigations have identified FGFR3 signaling as a key regulator of chondrocyte and osteoblast function both during development and postnatally. In particular, absence of signaling through FGFR3 in the joints of Fgfr3−/− mice leads to premature cartilage degeneration and early arthritis.16 These degenerative changes were accompanied by increased expression of MMP13 and type X collagen, cellular hypertrophy, and loss of proteoglycan at the articular surface. One of the key ligands of FGFR3 signaling appears to be FGF18 (for review, see Haque et al17). In the postnatal joint, FGF18 has significant anabolic effects on cartilage metabolism. The efficacy of FGF18 was tested in a rat meniscal tear model of OA.18 Intra-articular injection of FGF18 induced a dose-dependent increase in cartilage formation and a reduction in cartilage degeneration scores in the medial tibial plateau of OA rats. It is important to note that this effect was seen only in OA joints, not in normal rat joints, suggesting that this effect may be a specific response to tissue injury. It is interesting to note that at the molecular level, this joint protective effect may be due in part to its interaction with other signaling pathways such as BMP signaling by repressing noggin, a BMP antagonist.19 These findings, combined with enhanced understanding of FGF18 signaling in postnatal cartilage and bone biology, led to the development of local treatment with FGF18 and the design of early-phase clinical trials (clinicaltrials.gov) in patients with cartilage lesions and in those with OA of the knee.
Wnt Signaling
Recent findings indicate a critical role for Wnt signaling in cartilage and bone biology, with specific relevance to osteoporosis and osteoarthritis (for review, see Luyten et al20). Most available data provide circumstantial and/or direct evidence, both in vivo and in vitro, that activation of Wnt/β-catenin signaling leads to reprogramming of articular chondrocytes toward catabolism or loss of their stable phenotype, with subsequent loss of articular cartilage tissue structure and function. Frzb−/− mice lacking this Wnt antagonist show increased activity of the Wnt signaling pathway, leading to increased bone stiffness and enhanced cartilage damage.21 It is important to note that upon induction of joint changes reminiscent of OA by enzymatic treatment (papain-induced osteoarthritis), by accelerated instability (collagenase-induced ligament and meniscal damage), or by inflammation (methylated bovine serum albumin [mBSA]-induced monoarthritis), Frzb−/− mice showed greater cartilage loss than their wild-type counterparts.22 Increased cartilage damage in Frzb−/− mice was associated with higher levels of β-catenin–dependent Wnt signaling and with higher expression levels of matrix metalloproteinase 3. It was also demonstrated that FRZB can inhibit directly matrix metalloproteinase 3, probably through the netrin domain, indicating the potential complexity of the underlying mechanisms of observed phenomena. The role of Wnt signaling as a key regulatory pathway in postnatal joint biology and joint remodeling in chronic arthritis was further highlighted by the findings of Diarra and co-workers.23 Through inhibition of DKK1, an antagonist of the canonical Wnt signaling pathway, in a transgenic mouse model of TNF-driven inflammatory arthritis, part of the bone destructive effect was reversed. In view of the complexity of Wnt signaling and its potential downstream effects, it is important to further study the specific roles of canonical and noncanonical Wnt signaling in cartilage, bone, and the osteochondral junction, allowing Wnt-activated pathways and their components to become separate targets for therapeutic intervention. In view of this, recent findings of specific upregulation of Wnt16 in postinjury joint cartilage and in OA are of interest and open the opportunity for joint-specific Wnt targeting.24
Other Potential Anabolic Treatments
Other targets besides skeletal developmental pathways are signaling molecules that play a critical role in postnatal joint tissue homeostasis and turnover. These proteins/pathways can be regarded as potential postnatal anabolic agents contributing to the restoration of joint homeostasis. In this regard, the growth hormone (GH)/insulin-like growth factor (IGF) axis appears to be of interest. In particular, IGF-I has been reported to be critical in the maintenance of homeostasis of articular cartilage explants ex vivo.25 Further evidence of its anabolic effect in in vivo models has led to the early clinical development of intra-articular treatments with IGF-I in gonarthrosis, although no recent reports of ongoing clinical trials have been found. Alternatively, successful local delivery via gene therapy has been reported.26
Furthermore, it was reported that systemic administration of GH in horses may be beneficial for joint/articular cartilage biology, as it increases IGF-I levels in synovial fluid.27 Improved formulations of GH have been explored to improve duration and effect size in synovial joints.28
A relationship between levels of IGF-I and osteoarthritis has been suggested, further suggesting a potential benefit of targeting the GH/IGF-I axis, particularly in OA. However, data so far are not conclusive, and further systematic analysis of the hypothalamic-pituitary axis, including growth hormone, IGF-I, and somatostatin, is required.29 Taken together, the potential of GH/IGF-I as anabolic factors for joint repair and in particular their beneficial effects on articular cartilage metabolism have been recognized for a long time, but data supporting their clinical use and impact on joint biology in patients remain to be further explored.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree