Fig. 10.1
(a) Surface anatomy of the knee in resisted extension. (b) Anatomical depiction of the underlying knee extensor mechanism. Part (b) was published in DeLee and Drez’s Orthopaedic Sports Medicine, 3rd Edition, DeLee JC, Drez D, Miller MD, editors. Chapter 22: Patella, p. 1533–77, Copyright Saunders, an imprint of Elsevier (2010)
The vascular supply of the quadriceps tendon is of particular importance and has been described in detail [17]. The blood supply of the tendon consists of medial, lateral, and peripatellar arcades branching from muscular perforators of the deep and superficial femoral arteries, the descending genicular artery branch of the superficial femoral artery, and the superior medial and superior lateral genicular arteries from the popliteal artery. The tendon possesses an oval-shaped area of relative hypovascularity in its central, deep portion between 1 and 2 cm proximal to the superior pole of the patella [17]. This vascular watershed region is clinically important as is plays a role in rupture risk and tendon healing potential.
Biomechanics
Within the extensor mechanism, the osseous patella acts as a fulcrum, increasing the extensor moment arm and thereby transmitting the longitudinal contractile force at a greater distance from the knee axis of rotation [13]. Force transmission through the knee extensor mechanism occurs in a complex but coordinated fashion. Huberti and colleagues demonstrated variability in the force ratio between the quadriceps tendon and patellar ligament with varying degrees of knee flexion, a concept they coined “the extensor mechanism force ratio” [18]. According to their biomechanical study, the predominant force lies within the patellar ligament at a knee flexion angle of 30°, whereas the forces are equal at 50° of flexion. The quadriceps tendon has a mechanical advantage at these smaller flexion angles, and the patellofemoral contact area is located at the distal end of the patella. With knee flexion beyond 90°, the force in the quadriceps tendon is greater than the force in the patellar ligament. The contact area on the patella shifts proximally with increasing knee flexion, giving the patellar tendon a mechanical advantage [18]. This, in turn, relates the position of knee flexion to the likelihood of tendon failure. At a position of knee flexion less than 45°, the patellar ligament is more likely to be injured. In positions greater than 45°, the quadriceps tendon is at higher risk for tensile failure.
There is a paucity of data concerning the absolute in vivo strength of the human quadriceps tendon and tensile loads within the tendon during common activities of walking, running, or jumping. In a cadaveric study of young males, the quadriceps tendon was capable of withstanding average loads of greater than 2,000 N prior to failure [19].
Etiology and Mechanism of Injury
The quadriceps tendon is infrequently the site of knee extensor mechanism disruption, as the mechanism most commonly fails through fracture of the patella [3]. The quadriceps tendon is remarkably resistant to rupture. In an early study by McMaster, the tensile strength and points of rupture of adult rabbit quadriceps tendons were evaluated [20]. In his study, approximately 50 % of a tendon’s fibers had to be severed for rupture to occur, even when subjected to extremely high forces. Rupture at the osteotendinous and musculotendinous junctions or through muscle substance occurred at lesser loads [3, 20]. These data suggest that healthy quadriceps tendons do not rupture and some preexisting degeneration must be present for the mechanism to fail within in the tendon, an idea that has been widely accepted [3, 12, 17, 20–23].
Tendon degeneration may be secondary to overuse (particularly in the athletic population), anticipated changes with aging, tendon vascular disruption or compromise, or any of a multitude of systemic medical conditions. Kannus and Jozsa reported on the histological characteristics of biopsies of spontaneously ruptured tendon obtained during reparative procedures in 891 patients [23]. Characteristic histopathologic patterns in the ruptured tendons included hypoxic degenerative tendinopathy, mucoid degeneration, tendolipomatosis, and calcifying tendinopathy, either alone or in combination. In their study, no ruptured tendon was histologically normal. Comparatively, age-matched cadaveric controls were histologically normal in greater than 2/3 of specimens.
The hypovascular region of the quadriceps tendon may play a role in quadriceps tendon rupture etiology. Most tendon ruptures/strains occur at the musculotendinous or osteotendinous junction. The quadriceps tendon will most commonly rupture near its osteotendinous insertion and hypovascular region [17, 21, 24]. In a systematic review of quadriceps tendon ruptures, greater than 75 % of ruptures were identified within 2 cm of the superior pole of the patella [25]. Delayed healing in this location secondary to hypovascularity compromises the tendon’s ability to withstand repetitive microtrauma.
Systemic conditions altering the vascular supply and overall tendon integrity have been associated with tendon ruptures. Many of these conditions negatively affect the tendon microvasculature. This creates an additional insult to the already hypovascular region of quadriceps tendon and increases the tendon’s susceptibility to rupture. The quadriceps tendon is not exempt from the systemic microvascular compromise induced by diabetes. Additionally, hyperparathyroidism, systemic lupus erythematosus, osteomalacia, and the use of steroids can also cause microscopic damage to the vascular supply [26, 27]. Renal disease and uremia can weaken the quadriceps mechanism by causing muscle fiber atrophy and changes within the collagen structure [28]. Synovitis and fibrosis may result from chronic inflammatory changes associated with rheumatoid and gouty arthritis [29, 30].
Systemic conditions/medications associated with quadriceps tendon rupture include the following:
Diabetes
Hyperparathyroidism
Systemic lupus erythematosus
Gout
Pseudogout
Uremia
Rheumatoid arthritis
Steroids
Fluoroquinolones
Of particular importance in evaluation of the athletic population, anabolic steroid use has been shown to alter the structural and mechanical properties of tendon in animal studies [31, 32]. Several case reports have linked anabolic steroid usage to tendon rupture, including a case of bilateral quadriceps tendon ruptures [33, 34]. While a definitive cause–effect relationship has not been established, animal studies suggest the deleterious effects of their usage on tendon health.
When quadriceps tendon ruptures do occur, it is almost uniformly indirect in nature. While exceedingly rare, reports of rupture resulting from direct trauma and laceration are also present in the literature [3, 24]. The rupture most commonly occurs as the result of an eccentric contraction of the extensor mechanism against a sudden load of body weight with the foot planted and knee flexed [3, 8, 12, 21, 35]. This eccentric contraction is commonplace in many sporting activities and occurs any time an athlete lands from a position of elevation or performs the lowering phase of a squatting maneuver (Fig. 10.2a–h).


Fig. 10.2
(a–h) Sequence of landing from position of height. Note the eccentric body weight load on the quadriceps with the foot planted and knee flexed
Clinical Presentation
A rupture of the quadriceps tendon is often a clinical diagnosis based on history and physical examination. Patients will commonly present with the triad of acute onset knee pain, inability to actively extend the knee, and a suprapatellar gap (Fig. 10.3) [3, 24, 27, 36]. This triad is commonly accompanied by a report of a fall or sense of knee instability following the acute onset of knee pain. The pain is often intense and tearing in nature. On physical examination, anterior knee edema is often present, and a gap in the tendon may be appreciated with palpation. The patient is unable to actively extend the involved knee and experiences difficulty maintaining a straight leg raise against gravity.
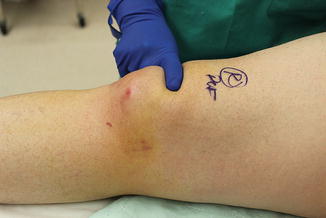

Fig. 10.3
Palpation of the ruptured quadriceps tendon demonstrates a palpable suprapatellar gap
With a partial tear or a complete tear with an intact patellar retinaculum, the patient may retain the ability to hold straight leg raise against gravity and extend the knee, albeit with much less force than the non-involved limb. Active knee flexion is retained. If initial presentation is not obvious or severe pain precludes adequate examination, knee aspiration and intra-articular anesthetic injection can help relieve the pain and allow the physician to more accurately assess the extensor mechanism. Despite the apparent simplicity in presentation and clinic diagnosis, the aforementioned confounders may mask or delay the diagnosis of a quadriceps tendon rupture. Delays of days to months and overall misdiagnosis rates ranging from 10 to 50 % have been reported in the literature [12, 37, 38].
Imaging
Although the diagnosis of quadriceps tendon rupture is usually evident following physical examination, further workup with diagnostic imaging is recommended for confirmation and identification of concomitant knee pathology. At a minimum, biplanar radiographs of the knee should be obtained for evaluation of the osseous knee structures and relative location of the patella. The lateral knee radiograph is particularly useful in the characterization of extensor mechanism disruptions. Quadriceps tendon ruptures may be identified by disruption of the tendon’s shadow [38]. The relative position of the patella can be evaluated by the previously detailed Insall-Salvati [39] or Blackburne-Peel [40] ratios. Of the two, the Blackburne-Peel ratio has been shown to be the most reproducible and consistent measure of patellar height [41]. The lateral radiograph and these defined ratios will commonly demonstrate a low-lying patella (patella baja). It should be noted, however, that a low-lying patella and ratio measures outside the normal reported range are not mandatory diagnostic prerequisites for quadriceps tendon ruptures.
Ultrasonography and magnetic resonance imaging (MRI) are two additional useful imaging modalities in the diagnosis of quadriceps tendon ruptures. In combination with physical examination, ultrasound has been shown to be a highly sensitive and specific means of quadriceps tendon rupture assessment [42–44]. Complete tears will demonstrate free end tendon fibers with intervening hypoechoic to anechoic area. The reliability of ultrasound remains operator dependent. While ultrasound results may be obscured by hematoma or edema associated with the rupture, MRI consistently and accurately depicts the injury and its location (Fig. 10.4) [43, 45, 46]. Perhaps the greatest benefit of MRI is its ability to identify concomitant knee pathology, although this occurs infrequently in disruptions of the knee extensor mechanism [47].
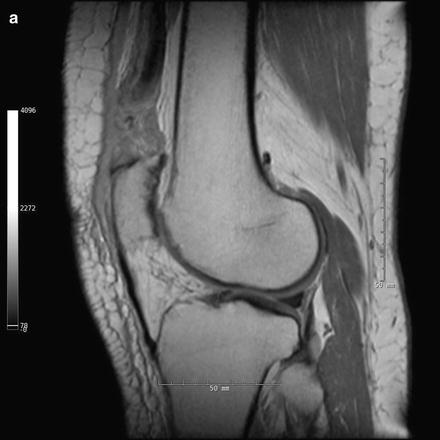
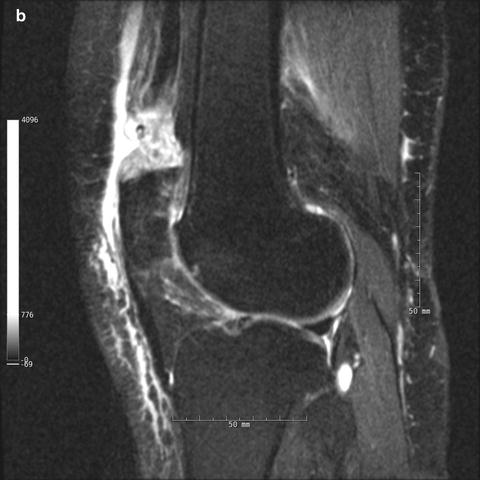


Fig. 10.4
(a) Sagittal proton-density turbo spin-echo MRI of right quadriceps tendon rupture. (b) Sagittal proton-density turbo spin-echo fat-saturated MRI of right quadriceps tendon rupture. Note the anterior effusion, complete discontinuity of the quadriceps tendon, and wavy appearance of the continuous patellar tendon
Treatment
The treatment strategy varies based on the presence or absence of a complete rupture and the chronicity of the rupture.
Partial
A conservative, nonoperative approach is the initial management of choice when a diagnosis of partial rupture is made. Traumatic hemarthrosis and effusion have been shown to decrease quadriceps strength [48]. Early treatment should thus consist of aggressive treatment of knee effusion with ice, compression, anti-inflammatory medication, and possibly knee aspiration to evacuate the hemarthrosis and promote rehabilitation. Operative treatment may be indicated if nonoperative management fails to provide resolution of symptoms. If one or more of the tendon layers are intact, partial ruptures can be treated with excision of the scar tissue and side-to-side closure of the tendon [49].
Complete
Weakness and long-term disability associated with nonoperative management of complete quadriceps tendon ruptures have made operative repair of the ruptured tendon the treatment strategy of choice. When the diagnosis of a complete quadriceps tendon is made, prompt referral to an operative orthopedic surgeon is recommended as delay in operative repair may result in poorer outcomes secondary to retraction of the proximal tendon and atrophy of the quadriceps musculature [6, 50, 51].
The basic tenets of operative repair of quadriceps tendon ruptures including the use of suture to approximate the tendon to and through its patellar remnant have remained unchanged since the procedure was first reported in the 1880s [3]. Based on these tenets, numerous effective surgical strategies have been derived for the management of this injury; no technique has been proven to produce superior results [3, 7, 49–51]. A randomized, controlled trial comparing outcomes of quadriceps tendon ruptures repaired by the various techniques has not been completed. The type of surgical treatment is largely dependent on the location of the rupture within the tendon and surgeon preference.
For surgical intervention, the patient is placed supine on an operating room table, frequently with a bump under the involved hip to prevent external rotation of the extremity. The anatomic surgical approach shows little variability across treatment types and consists of an approximately 10 cm midline longitudinal incision over the anterior knee to expose the extensor mechanism. If hematoma is present, this is evacuated to allow visualization of the ruptured tendon ends. The tendon ends are debrided of grossly degenerative tissue to prepare them for repair.
For mid-substance tears, an end-to-end primary repair with heavy nonabsorbable suture is an effective treatment strategy. Using a No. 2 or No. 5 nonabsorbable suture, continuous running stitches are sewn up and down the lateral borders of the free proximal and distal tendon ends in a locking fashion. This is repeated along the medial borders of the free ends of the ruptured tendon, leaving four free suture ends from the proximal and distal tendon remnants. The corresponding sutures are then tied with the knee in full extension, resulting in four knots at the site of tendon rupture. The repair may be augmented with additional simple interrupted sutures at the rupture site. The medial and lateral patellar retinaculum are then reapproximated with another heavy nonabsorbable suture to complete the repair. The stability of repair and patellar tracking are then tested by taking the repaired knee through gentle range of motion.
Ruptures at or near the osteotendinous junction typically require a slightly more advanced repair strategy. Suture anchors at the superior pole of the patella or a series of three drill holes longitudinally through the patella may provide appropriate recreation of the quadriceps tendon portion of the extensor mechanism (Fig. 10.5). The transosseous technique is most commonly utilized. Two separate heavy nonabsorbable sutures are passed through the medial and lateral portions of the tendon in a locking fashion as described above, resulting in four equal-length tendon strands at the free tendon edge. A trough is made in the superior margin of the patella with a high-speed burr, thereby creating a bleeding bone bed to achieve tendon to bone healing. The trough must be created horizontally in the midpoint of the superior pole of the patella to prevent abnormal tilt when the tendon is reapproximated. A 2.0 mm drill is then used to create three parallel transosseous patella drill holes with starting points in the trough 1 cm apart. It is important that the drill holes are parallel to the patellar articular surface and each other. Convergence should be prevented as this may cause failure of the repair by suture pullout through the bone bridge. The sutures are shuttled through the patellar holes with the use of a Hewson suture passer so that one suture end passes through each of the lateral and medial holes and two pass through the medial hole. Alternatively, Beath pins can be used to create holes and shuttle the sutures. The sutures are tied distally with the knee in full extension, resulting in two knots at the inferior pole of the patella (Fig. 10.6). The medial and lateral patellar retinaculum are then reapproximated with a heavy nonabsorbable suture. Following recreation of the extensor mechanism, the knee is gently ranged from 0 to 90° to ensure absence of gapping, appropriate tension, and appropriate patellar tracking. The repair may be augmented with a cerclage suture [52]. In this technique, a heavy nonabsorbable “relaxing suture” is passed through the quadriceps tendon proximal to the locking stitches, woven down through the medial and lateral retinaculum, passed below the distal pole of the patella, and tied with the knee in 30° of flexion to reinforce the repair. Alternative approaches to repair including the use of a Leeds-Keio ligament [53], Dacron vascular graft [54], or Mersilene tape [55] have been described but are used infrequently.
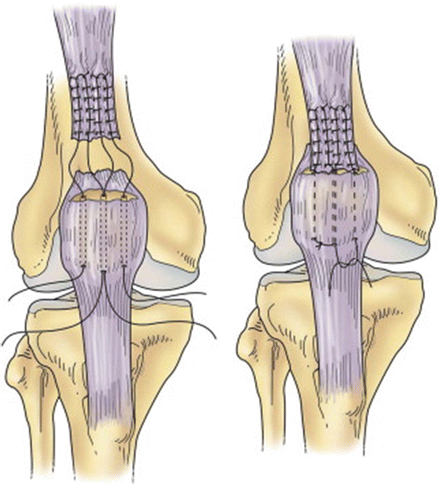
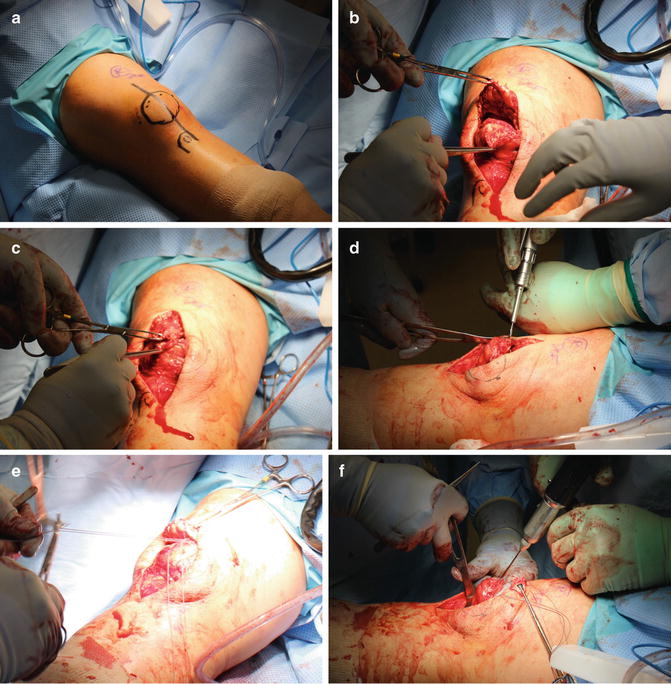
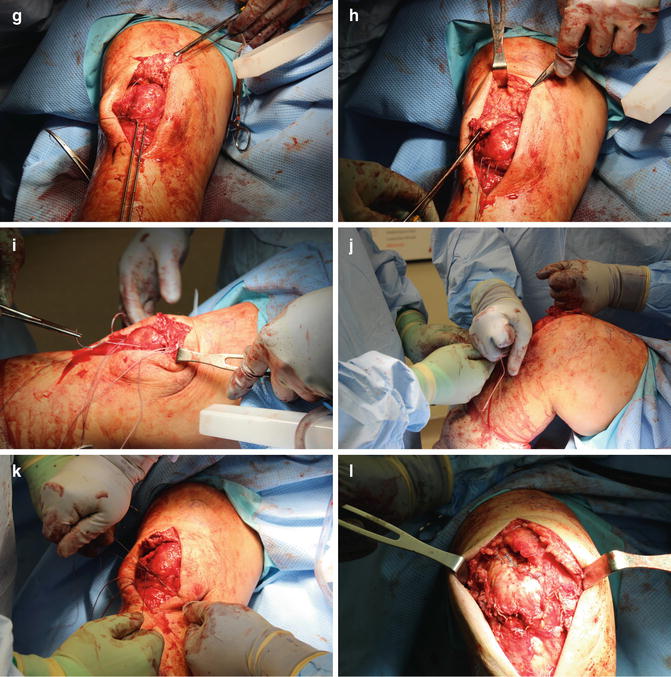

Fig. 10.5
Depiction of surgical repair with suture through transosseous tunnels. This figure was published in Insall and Scott Surgery of the Knee, 5th Edition, Seidenstein AD, Farrell CM, Scuderi GR, Easley ME, Chapter 66: Quadriceps and patellar tendon disruption, p. 696–710, Copyright Churchill Livingstone (2012)


Fig. 10.6
(a) Planned operative incision with outline of patella and tibial tubercle. (b) Debrided edges of the ruptured quadriceps tendon reflected. (c) Debrided edges of the ruptured quadriceps tendon opposed. (d) Creation of the trough in the superior pole of patella with burr. (e) Tendon is sutured with heavy nonabsorbable suture in locking fashion along its medial and lateral borders. (f) Beath needle is drilled through the patella parallel to its articular surface. (g) Beath needles in parallel configuration with 1 cm spread (lateral suture has been threaded through drilled hole). (h) Remaining sutures shuttled through the drilled holes. Note the reapproximation of tendon to the superior pole of patella. (i) Cerclage “relaxing suture” sewn through medial retinaculum. (j) Cerclage “relaxing suture” tied with knee in flexion. (k) Sutures shuttled through the patella drill holes are tied at inferior pole of patella with knee in extension. (l) Reapproximation of the quadriceps tendon
Chronic
Unfortunately, a delayed or missed diagnosis of a quadriceps tendon is not a rare occurrence [12, 37, 38]. Management of this subset of tears requires a different treatment approach as scarring and tendon retraction may prohibit a direct end-to-end or tendon-bone repair of the tendon. Elevation of the quadriceps from the femoral shaft provides additional length to bridge small retraction gaps, but scarring and large gaps often limits its utility. The inverted V turndown flap techniques popularized by Scuderi and Codivilla can provide reinforcement and additional length to the chronic quadriceps tendon repair (Fig. 10.7) [24]. In this technique, the ends of the quadriceps tendon are first approximated with a heavy nonabsorbable suture. This is followed by the creation of an inverted V-shaped flap (partial thickness in Scuderi technique and full thickness in Codivilla technique) of the proximal quadriceps tendon with its base 1 cm proximal to the tendon tear. The flap is then folded distally so that the body of the V overlies the rupture site and the apex of the V is distal. In the full-thickness Codivilla technique, the proximal portion of the tendon is then repaired side to side to minimize compromise of the tendon.
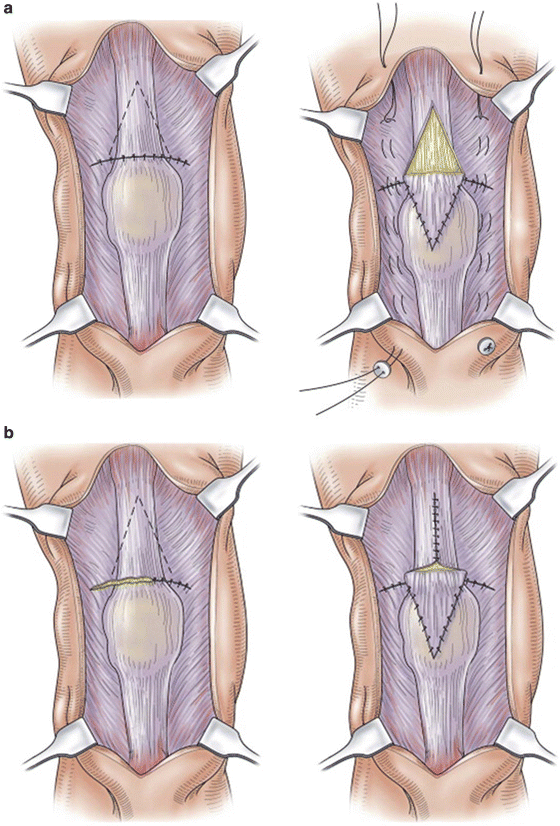

Fig. 10.7
(a) Scuderi technique. (b) Codivilla technique. These figures were published in Insall and Scott Surgery of the Knee, 5th Edition, Seidenstein AD, Farrell CM, Scuderi GR, Easley ME, Chapter 66: Quadriceps and patellar tendon disruption, p. 696–710, Copyright Churchill Livingstone (2012)
Rehabilitation
Exact rehabilitation protocols are variable from surgeon to surgeon and are determined by type of tear, surgeon experience, and intraoperative assessment of the repaired tendon. The first 3–6 weeks of rehabilitation for a partial quadriceps tendon injury consisted of immobilization with a hinged knee brace locked in extension and weight bearing as tolerated on the involved extremity. The second phase of management focuses on unlocking the knee brace, regaining knee flexion, and progression to full weight bearing. The final phase consists of continued focus on knee range of motion and strengthening with return to sport when both return to pre-injury levels, usually around 4–6 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







