39 Pregnancy in the Rheumatic Diseases
Potentially teratogenic medications should ideally be discontinued several months before conception.
In order for the “fetal semiallograft” to survive and grow during 9 months of exposure to the maternal immune system, the maternal immune system must undergo a complex modulation of the innate and humoral immune system, much of which is not well understood. Pregnancy had long been understood as a Th2-predominant condition, whereby a shift of helper T cells toward a Th2 dominant state, possibly induced by increasing levels of progesterone, is necessary to establish and maintain a normal pregnancy. This theory was consistent with earlier observations that systemic lupus erythematosus (SLE, a Th2-predominant disease) may be exacerbated by pregnancy, whereas Th1-mediated autoimmune diseases (rheumatoid arthritis [RA], multiple sclerosis, and psoriasis) appear to be characterized by clinical improvement during pregnancy.1 More recently, however, it is becoming increasingly clear that many more components of both the innate and adaptive immune systems are involved in normal pregnancy.2–5 Furthermore, many of the immunologic changes during pregnancy may be preferentially located at the maternal-fetal interface and may not be accurately sampled using peripheral blood. The trophoblast and placenta, once considered passive mediators of maternal-fetal immune trafficking, have been increasingly recognized as playing active roles in mediating inflammation, as well as in host defense.2 Taken together, it is evident that understanding the immune regulation of a healthy pregnancy remains elusive, much less understanding how pregnancy-related immunologic changes interplay with an abnormal immune system stemming from preexisting autoimmunity.
Systemic Lupus ErythematosUs
Pregnancy Outcomes
Retrospective studies of women with SLE have noted an increased rate of pregnancy loss when compared with healthy women. Among 203 women with SLE, 166 of their friends, and 177 of their relatives, pregnancy loss was twice as common (21% in SLE vs. 14% in friends and 8% in relatives, P < .0001) and preterm birth was three times more frequent (12% in SLE vs. 4% in friends and relatives, P < .0001).6 Prospective cohorts of SLE pregnancies report a more modest pregnancy loss rate, but these cohorts probably miss some early pregnancy losses that occur before presentation in the rheumatology clinic. What they notably show is a rate of stillbirth, typically defined as a pregnancy loss after 20 weeks gestation that is 5- to 10-fold higher than that of the general population (Figure 39-1). In a meta-analysis of 37 studies of SLE pregnancies (12 prospective and 25 retrospective), there is a higher than expected rate for miscarriage, stillbirth, and neonatal death (infant death at <28 days of life).7 Several studies have documented that increased SLE activity around the time of conception, hypertension, prior or current lupus nephritis, and antiphospholipid syndrome increase the risk for pregnancy loss by twofold to fourfold.
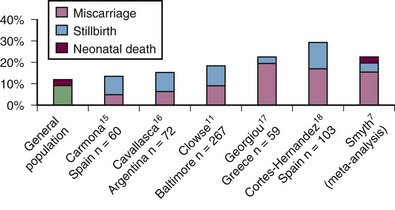
Figure 39-1 The rate of pregnancy loss in prospective cohorts of pregnancies in women with systemic lupus erythematosus (SLE).7,11,15–18 The miscarriage rate is typically not higher than reported in the general population, though prospective registries may miss early losses that occur before rheumatology visit. The risk of stillbirth is estimated at 1% in the United States but is between 5% and 8% in most prospective SLE pregnancy cohorts.
Preterm birth, defined as a birth before 37 weeks’ gestation, occurs in about one-third of SLE pregnancies (Figure 39-2). Among live births, the meta-analysis found that 39.4% of deliveries were preterm.7 The cause of preterm birth in the majority of these pregnancies has not been well quantified, but it is frequently induced because of maternal SLE disease activity, preeclampsia, slow fetal growth, or fetal distress. Spontaneous preterm birth, either prompted by contractions and preterm labor or premature rupture of membranes, is also increased in SLE. In one carefully collected prospective cohort of SLE pregnancies, half of the pregnancies that survived past 23 weeks’ gestation delivered preterm. Among these 33 preterm deliveries, 13 (39%) were due to premature rupture of membranes compared with 18% in a control population of preterm births (P = .001).8 The main predictor of preterm birth in women with SLE is increased disease activity during pregnancy.
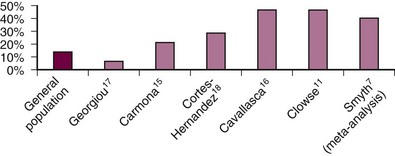
Figure 39-2 Preterm birth (delivery before 37 weeks’ gestation among live births) is increased in most prospective pregnancy cohorts.7,11,15–18
Preeclampsia is the syndrome of hypertension and proteinuria that occurs in the second half of pregnancy and resolves postpartum. The etiology is not entirely understood, but the current main hypothesis is that impaired trophoblast invasion of the uterus early in pregnancy results in poor development of the spiral arteries that deliver blood from the mother, through the placenta, and to the fetus. Later in pregnancies, these arteries are not able to expand to provide sufficient blood flow to the fetus, resulting in a cascade of endocrine, inflammatory, and endothelial changes that promotes maternal hypertension and proteinuria.9 In the general population, preeclampsia typically occurs in 5% to 8% of pregnancies and only 1/10 of these present before 34 weeks’ gestation. The risk for preeclampsia is increased if a woman has previously had preeclampsia, particularly if it occurred early in pregnancy and was severe (blood pressure over 160/110, proteinuria over 5 g/24 hr, or other severe organ manifestations). Also at risk are first pregnancies and pregnancies in women with obesity, diabetes, hypertension, age older than 40, and a family history of preeclampsia.9 Up to one quarter of pregnancies in women with SLE are complicated by either hypertension or preeclampsia.10 In a meta-analysis of SLE pregnancies, 7.4% reported preeclampsia, but the rate varied greatly between studies.7 Preeclampsia can be difficult to distinguish from active lupus nephritis. Other signs of SLE activity including arthritis, rash, low complement, or rising double-stranded deoxyribonucleic acid (dsDNA)-titer can help determine the etiology of hypertension and proteinuria. Treatment of these two conditions is different: immunosuppression for SLE and delivery for preeclampsia. In some situations, treatment for both is prudent and the ultimate determination of cause will be possible postpartum either through resolution of preeclampsia over the first month after delivery or renal biopsy for persistent disease.
Placental insufficiency, which leads to less blood and nutrient flow to the developing fetus, can lead to small babies. Definitions of this vary, but it can be measured both in utero via ultrasound and at birth by birth weight and is typically defined as a baby weighing less than the 10th percentile of expected weight for gestational age. In the meta-analysis of SLE pregnancies, 12.7% of babies were noted to be small. In an analysis of the Nationwide Inpatient Sample, which includes data for 20% of all deliveries in the United States, 5.6% of pregnancies in women with SLE were identified as intrauterine growth restricted, a rate that was 2.6-fold higher than for other pregnancies in the United States (P < .01).10 In the Hopkins Lupus Cohort, 23% of all live births weighed less than the 10th percentile for gestational age of delivery.11
Although up to 30% of women with SLE will have a flare during pregnancy, the majority of these are fairly mild and readily controlled.7,11 Several studies in the 1980s and 1990s tried to determine whether SLE worsens with pregnancy, but the conclusions were often conflicting. It seems that many patients will proceed through pregnancy without difficulty, but an important minority will suffer significant disease activity that can harm both the pregnancy and the woman’s own health and survival.
Significant SLE activity is associated with increased pregnancy loss and preterm birth. The degree of SLE activity is important—it appears that mild activity of the skin or joints will have minimal impact on pregnancy outcomes. Internal organ involvement including the kidneys, hematologic cell lines, serositis, and the central nervous system (CNS) is more highly associated with pregnancy morbidity. Women with highly active SLE around the time of conception have an estimated 40% risk of pregnancy loss.11,12 This applies to women with high overall disease activity, with isolated proteinuria greater than 500 mg/24 hr, or isolated thrombocytopenia.12 Disease activity in the second and third trimesters is less often associated with pregnancy loss but can double the rate of preterm delivery.11
The most important predictors of SLE activity in pregnancy are increased SLE activity in the 6 months before conception, the frequency of SLE flare in the years before pregnancy, and the cessation of immunosuppressive medications. Among women with high-activity SLE in the 6 months before conception, 58% had high-activity SLE during pregnancy. On the other hand, of women with relatively quiet SLE before pregnancy, only 8% had high activity in pregnancy.11 The cessation of hydroxychloroquine (HCQ) before or concurrent with conception is associated with a high rate of SLE flare, particularly in the second and third trimesters. Because these flares are predominantly in the skin and joints or manifest as fatigue, they do not have a statistically significant impact on pregnancy outcomes. They can, however, lead to significant discomfort and the use of higher doses of corticosteroids during pregnancy. Stopping immunosuppressants including mycophenolate mofetil or azathioprine because of pregnancy may lead to a flaring of SLE activity, particularly renal or hematologic, that could lead to significant pregnancy morbidity. For this reason, women who require these drugs to maintain quiet SLE activity before pregnancy will likely benefit from continuing azathioprine during pregnancy (see further discussion in Medications in Pregnancy and Lactation later).
Lupus nephritis, whether active or quiescent during pregnancy, affects pregnancy outcomes. Several prospective studies of lupus nephritis in pregnancy document that women with prior lupus nephritis have a higher rate of pregnancy loss, preterm birth, and preeclampsia than other women with and without SLE. Active lupus nephritis during pregnancy increases the pregnancy loss and preterm birth rates by twofold to threefold.13,14 Renal failure is a rare complication of active lupus nephritis in pregnancy.
Maternal death is 20-fold more common among SLE pregnancies than other pregnancies, but it is fortunately rare.8 When compared with the annual risk of death in all women with SLE (ranging between 0.5% and 1% of all women, depending on the study sample), the risk of death in pregnancy for women with SLE is lower (0.3%).8 This is likely because women with the most severe SLE, who are at the highest risk for death, avoid pregnancy. Reported causes of maternal death in women with SLE include thrombosis, in particular pulmonary embolism, and infection. Although risk factors for maternal death have not been studied due to the rarity of this event, patients at higher risk for death include those with prior major thrombosis; severe pulmonary hypertension, lung, or renal disease; and women requiring high-dose immunosuppression.
1. Time pregnancy to coincide with periods of disease quiescence. At least 6 months of quiet SLE is required to decrease the risk of disease flare and adverse pregnancy outcomes. Accomplishing this requires discussing and prescribing effective contraception to women with significant SLE activity to avoid conception. Active SLE does not impair fertility.
2. Avoid teratogenic medications, but continue immunosuppression when needed. The adverse effects of highly active SLE generally outweigh the teratogenic effects of many SLE medications. Women taking HCQ before conception should continue this drug to avoid an SLE flare. Women who are taking mycophenolate mofetil or cyclophosphamide to control SLE activity should either avoid pregnancy or switch to azathioprine before pregnancy (or when pregnancy is discovered). Discontinue angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), but continue blood pressure control with calcium channel blockers and labetalol.
3. Use prednisone to treat SLE flare, but do not use prophylactically in a woman with quiet disease.
4. Start women with SLE on low-dose aspirin (81 or 100 mg a day) to decrease the risk of maternal thrombosis, preeclampsia, and preterm delivery.
5. Have all women followed by, or at least seen in consultation by, a high-risk obstetrician (also called maternal-fetal medicine).
6. Continue close rheumatology follow-up for women with SLE during pregnancy. Do not assume that the obstetrician will continue to monitor for or will be able to identify SLE activity.
Rheumatoid Arthritis
RA is a female-predominant disease, affecting women more than men in a 3 : 1 ratio. The prevalence of RA in the United States is approximately 1.4% with a median age of onset of 59 years. The prevalence of women of childbearing age in the United Kingdom (data on prevalence in the United States are not available) is estimated to be 1 to 2/1000 of women between ages 16 and 44.19 A recent study estimated that approximately 1400 women with RA gave birth in the United States in 2002.20,21
Fertility
Although it has been apparent for many decades that women with RA tend to have fewer children or are more likely to be nulliparous than control populations, there is a paucity of data looking at RA associated with fertility. Katz22 studied pregnancy patterns and decisions regarding childbearing in a group of 411 women with RA in 1999. Results showed that family sizes were smaller among women who developed RA before having any children or to completing childbearing. Approximately 30% of respondents who developed RA during the childbearing years reported that the diagnosis affected their decision to have additional children, and 12% reported being advised by a provider to limit family size. From these data, it appears that reduced numbers of children among women with RA may be related to choice rather than to reduced fertility or increased pregnancy loss.22 Reduced sexual activity due to functional limitations from the disease are a further, nonbiologic cause of lower birth rates due to reduced opportunity for conception.23
Although several disease-modifying antirheumatic drugs (DMARDs) may assert teratogenic effects on the fetus,24 and nonsteroidal anti-inflammatory drugs (NSAIDs) have been associated with temporal and reversible infertility,25 most chronic medications taken to manage inflammatory arthritides have no known adverse effect on hormonal, ovarian, or endometrial function. Several studies have found that estrogen-containing oral contraceptives yield a protective effect or mildly ameliorating effect on RA, and increased use of oral contraceptive pills among women with RA may further contribute to lower birth rates. These medication issues are easily overcome in women with RA who desire a pregnancy. However, it is much more difficult to formally study biologic infertility in this population. Few studies have been able to disentangle true inability to conceive a child from the myriad of other reasons for which women with RA may have fewer pregnancies. There has been a suggestion of reduced ovulatory function among women with RA in a small study of menstruating women,26 but this has yet to be followed up with larger studies. A recent population-based study from Norway has found that women with RA used assisted reproduction technologies more than age-matched healthy women.27 Similar findings were seen in a case-control study of pregnant women in the Netherlands (17.8% RA patients used assisted reproductive techniques compared with 3% in healthy women).28
Pregnancy Outcomes
In the absence of exposure to potentially teratogenic medications (including methotrexate, leflunomide, and mycophenolate mofetil [MMF]), earlier observations showed that maternal-fetal outcomes did not appear to be changed by maternal RA.20 However, more recent studies have shown that there are, in fact, some differences in these outcomes comparing women with RA with the general population. Several recently published population-based studies have documented an increased risk of preterm birth (<37 weeks’ gestational age) and small for gestational age (SGA, <10th percentile weight for gestational age) among women with RA compared with healthy women (Table 39-1). Although the odds ratios are generally less than 2.0, the consistency of reports over several different populations suggests a true association between a diagnosis of RA and adverse fetal outcomes. Because most of these studies are population based, relying on administrative data, they do not contain data regarding medication use or disease activity before conception or during gestation. Two prospective cohort studies of pregnant women with RA compared with healthy pregnant women have demonstrated an inverse association between disease activity during the third trimester of pregnancy and birth weight.28,29 One study from the Netherlands found a linear relationship between third-trimester disease activity (assessed by a modified DAS28-CRP) and birthweight in a multivariate model that adjusted for gestational age, maternal smoking, maternal age, education, parity, and prednisone use.28 A second study, from the United Kingdom, found that women with active disease during the third trimester had lower birth weight than women with RA in remission; women with inactive RA had birth outcomes indistinguishable from healthy control women.29 Together, these data suggest that RA is associated with higher risks of preterm delivery and lower birth weight infants; however, this may be mitigated by achieving good control of disease activity during at least the third trimester of pregnancy.
Disease Outcomes
Since the initial report by Hench in 1938,33 it has been thought that RA disease activity improves during pregnancy. In reviewing the majority of the data looking at disease activity during pregnancy, it appears that approximately 75% of patients will have some degree of improvement.34,35 This may occur as early as the first trimester and continue through the end of pregnancy. The exact underlying immunologic mechanism as to why this occurs is unclear. However, more recent reports have suggested that this improvement is actually not as dramatic as was once perceived. De Man and colleagues looked prospectively at 84 patients with RA and determined improvement and deterioration by the DAS28 changes.36 For women who started the pregnancy with moderate disease activity, only 48% had a moderate improvement response. Those with low disease activity during the first trimester tended to remain stable. Only 27% of the patients as a whole were in remission by the third trimester. In comparison, Barrett37 and Nelson and colleagues35 in previous studies showed that 16% and 39%, respectively, were in remission during the third trimester (though different definitions of remission were used). Unfortunately, no clinical or laboratory predictors have been found to reliably predict the disease course during pregnancy. Conflicting reports have been published looking at the degree of maternal-fetal human leukocyte antigen (HLA) class II mismatch and maternal RA disease activity.35,37–40 The majority of studies have confirmed that a higher degree of maternal-fetal HLA mismatch is associated with improved disease activity during pregnancy35,38,39; however, a study of 110 maternal-fetal pairs failed to find such an association.40 More recently, a prospective study of 118 pregnant women with RA from the Netherlands found that women who were seronegative for rheumatoid factor or anticyclic citrullinated peptide had a greater likelihood of improvement in disease activity during pregnancy.41 This has yet to be studied in other populations.
Despite a wide range of disease activity during pregnancy, the majority of studies have shown that RA may worsen or flare in the postpartum period.34,36,37,41 In addition, a recent study has demonstrated an increase in the incidence of RA during the first 24 months following delivery compared with 25 to 48 weeks’ postpartum among premenopausal women in Norway,42 suggesting that either the incidence of RA peaks in the postpartum months or pregnancy may delay the onset of RA in susceptible individuals.
Ways to Improve Outcomes
Monitoring disease activity during pregnancy can be tricky because distinguishing underlying activity of RA from normal changes during pregnancy is often not clear. Both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are markers of systemic inflammation that are often used to follow disease activity in rheumatic disease. Unfortunately, both these levels can fluctuate in pregnancy with more dramatic increases seen in ESR rather than CRP.36 Therefore these levels must be interpreted with caution in women with RA. Furthermore, clinical changes of pregnancy such as fatigue, swelling (especially of the distal extremities), and carpal tunnel syndrome are commonly seen in pregnancy. Careful examination of the wrist and assessment of neurologic symptoms are important to distinguish carpal tunnel syndrome from RA flare.
Given the growing evidence that active disease during pregnancy is associated with increased risks of adverse fetal outcomes, it is important to achieve control of disease activity before conception and during pregnancy whenever possible. In the ideal situation, women with RA considering pregnancy should aim to achieve good control of disease after discontinuation of medications with teratogenic potential, using medications considered safer during pregnancy if necessary to reduce inflammation. Determining the right medications for a pregnant patient can be challenging and requires an individualized approach. Certain medications are strictly contraindicated during pregnancy and should always be avoided due to teratogenic potential: methotrexate and leflunomide. Additionally, these agents should be discontinued immediately on the discovery of pregnancy in a woman receiving these medications (leflunomide will require an additional washout with cholestyramine).43 If a disease-modifying agent is required to control chronically active disease during an anticipated or confirmed pregnancy, plaquenil, sulfasalazine (SSZ), and/or azathioprine carry the fewest risks to the developing fetus and are generally considered relatively safe.44 However, these agents may have limited efficacy for moderate to severely active disease and may be insufficient to control disease during pregnancy. The use or continuation of anti–tumor necrosis factor (TNF) agents during pregnancy has been more controversial. Some studies have suggested an increased risk for congenital abnormalities, whereas others have not.45 Cumulatively, the data seem to show that exposure to anti-TNF agents in pregnancy does not seem to increase risk of miscarriage, preterm delivery, or congenital malformations.46 In cases of severe inflammatory disease before or during pregnancy, TNF inhibitors may be used with caution, provided that the mother is aware of and comfortable with the risk-to-benefit ratio.
For acute flares of disease, use of glucocorticosteroids is the best option because they have a rapid onset of action and can be titrated to the level of disease activity. There is vast experience with glucocorticoids during pregnancy for many indications. Prednisone is perhaps the most ideal choice for the treatment of maternal disease because a very small amount of active drug will enter the fetal circulation.44 In fact, some women may even require regular glucocorticoid use if background DMARD therapy is ineffective. The goal should always be to minimize the dose as best as possible and to prescribe adequate calcium and vitamin D supplementation during use. Isolated joints may be best managed through direct local injection of steroids, thus avoiding significant systemic exposure to the drug. Although intermittent NSAID use appears to be safe, there are some risks to regular use, particularly during the latter half of pregnancy because it can be associated with premature closure of the ductus arteriosus.44
Scleroderma
The hallmarks of scleroderma, vasculopathy and fibrosis, complicate surprisingly few numbers of pregnancies in women with this disease. Pregnancies are relatively rare in women with scleroderma, primarily because the onset of the disease is often after a woman has completed her family. About 200 pregnancies in women with scleroderma have been reported in the literature, however, with largely good results. The rate of miscarriage is about 15%, which is 50% higher than in the general population.47 Stillbirths, however, are rare. Preterm birth rates range from 25% to 40%, dependent on the degree of internal organ damage.47 Reports of preeclampsia are rare, but in a nationwide database of pregnancies, 23% of the 504 with scleroderma had hypertensive complications, including preeclampsia.48
To maintain pregnancy, a woman’s body must make major hemodynamic changes including an increase in blood volume of 50%, increase in cardiac output, and decreased vascular resistance.49 These adaptations may be hindered by underlying vasculopathy in women with scleroderma. Pulmonary hypertension, in particular, can both limit pregnancy success and put the woman at significant risk for death from hemodynamic collapse. Pregnancy in a woman with pulmonary hypertension carries a maternal mortality rate of 17% to 33% with deaths caused primarily by right ventricular failure or pulmonary embolism.50 The risk for death is greatest around the time of delivery and in the weeks postpartum. Neonatal or fetal death after 22 weeks’ gestation occurred in 7% to 13% of pregnancies complicated by pulmonary hypertension, about one-third had intrauterine growth restriction, and more than 85% delivered preterm.50 Medical treatment of pulmonary hypertension may include prostacyclin, nitric oxide, and calcium channel blockers. Due to the high maternal and fetal morbidity and mortality, pulmonary hypertension is considered a contraindication to pregnancy.50
Scleroderma renal crisis, though rare in pregnancy (occurring in 2% to 3% of scleroderma pregnancies), can be catastrophic.47 It can be difficult to distinguish from preeclampsia, both presenting with hypertension, proteinuria, and in severe cases hemolysis and thrombocytopenia. Women at particular risk for this complication include those with a prior history of it and those with recent onset of rapidly progressive diffuse disease. If the pregnancy is near term, delivery followed by aggressive ACE inhibitor therapy is indicated. If not near term, treatment with ACE inhibitors should be weighed. These drugs carry significant risk for permanent and fatal renal disease in the fetus but are also the essential treatment for scleroderma renal crisis.47
Gastroesophageal reflux disease often worsens in pregnancy. Raynaud’s phenomenon, however, often improves due to decreased vascular resistance.47 Skin disease does not seem to change significantly in pregnancy.47 The peripheral edema of pregnancy may be particularly uncomfortable, however, for women with diffuse scleroderma.
1. Women with pulmonary hypertension, a history of scleroderma renal crisis, or severe interstitial lung disease should be made aware of the significant risk to their life, health, and the survival of a pregnancy due to their disease.
2. Women without known pulmonary hypertension should undergo screening with an echocardiogram and pulmonary function testing before or early in pregnancy to identify previously subclinical disease.
3. Women taking ACE inhibitors or angiotensin-receptor blockers should discontinue these prior to pregnancy, if possible.
4. Due to the high risk of preterm birth and preeclampsia, a daily dose of low-dose aspirin should be considered.
5. Women with scleroderma should be followed closely in pregnancy by rheumatology and a high-risk obstetrical team. In addition, cardiology or pulmonologists may need to be involved on the basis of the degree of cardiopulmonary disease.
6. Repeated screening for intrauterine growth restriction should be performed in the third trimester.
Psoriatic Arthritis
Psoriatic arthritis (PsA) is a chronic destructive autoimmune arthropathy often associated with the presence of psoriasis. Psoriasis is a chronic inflammatory skin condition manifested by hypertrophic erythematosus plaques with overlying scale that can be quite disfiguring and functionally limiting for affected patients. Both psoriasis and PsA affect men and women equally; the average age of onset of psoriasis is in the second and third decades with PsA often occurring approximately 10 years later among the 20% who will go on to develop PsA.51
Pregnancy and Disease Outcomes
Outcomes data for women with PsA are scarce52; however, preconception counseling for patients with these conditions is similar to those with RA. They should have a good understanding of drug toxicities, side effects, and benefits of use as detailed earlier. In addition, it appears as though women can expect some degree of improvement in their arthritis symptoms during pregnancy. At least half of women show some resolution of their arthritis in case series reported to date.53,54 However, the degree of improvement has not yet been elucidated because more recent data reviewing pregnant PsA cohorts have not occurred. Furthermore, most case series and reports of pregnancy in PsA have occurred before the use of biologics and the era of better disease control, so it is difficult to say with certainty if these women actually clinically improve during as opposed to before pregnancy. As in RA, a postpartum flare often occurs within 3 months.53 Studies looking at pregnancy and fetal outcomes appear to show no increase in premature labor, preeclampsia, or adverse fetal outcomes.53,54
Ankylosing Spondylitis
Ankylosing spondylitis (AS) is a chronic autoimmune disease that most often affects the axial skeleton, as well as the hips and shoulders, that is often, but not always, associated with the HLA-B27 allele. In contrast to most autoimmune diseases, AS displays a male predominance in a ratio of 4 : 1. The overall prevalence of disease in Caucasian populations is approximately 0.03% to 0.1%.55 Prevalence estimates in women of childbearing age are not available, but the incidence in Rochester, Minnesota, is 3.6 to 6.4 cases per 100,000 person-years.56 Treatment options for AS are much more limited than for RA, and most therapies do not appear to abrogate the progressive spinal fusion that is characteristic of the disease. Because of the relatively low incidence of AS in women of childbearing potential, data regarding pregnancy experiences in this population are sparse.
Pregnancy Outcomes
Unlike SLE and RA, underlying maternal AS does not appear to be associated with increased risks of maternal or fetal morbidity. A questionnaire-based study of 649 women with AS in North America and Europe57 was performed looking at pregnancy outcomes. It was found that 15.1% ended with miscarriage, a rate not dissimilar to the general population. The overwhelming majority (93.2%) of cases delivered at term, but 58% delivered by cesarean section with AS, rather than other pregnancy-related conditions, as the stated indication. Neonates were healthy with a mean birth weight of 3339 g. Studies looking at pregnancy and fetal outcomes appear to show no increase in premature labor, preeclampsia, or adverse fetal outcomes.
Disease Outcomes
Most women with AS who become pregnant either remain stable or may have some clinical worsening of symptoms during the course of pregnancy. In addition, at least half will have some aggravation of symptoms in the postpartum period as well.54,57–59 In Ostensen and Husby’s questionnaire-based study of pregnancy in AS, disease activity during pregnancy was reported as unchanged in 33.2%, improved in 30.9% (which correlated with a history of peripheral arthritis), and worsened in 32.5% (which was mostly increased low back pain/hip pain after week 20). Sixty percent had a postpartum flare within 6 months after delivery.57 In a smaller study of nine patients with AS followed prospectively during pregnancy with validated disease activity measures (BASDAI scores, morning stiffness, patient global assessment), the majority had active disease during pregnancy, four had a decrease of 20% during pregnancy, and no remissions were found. Furthermore, women with AS had high pain scores throughout pregnancy when compared with women with RA, with resultant increased utilization of NSAIDs and paracetamol.60
Special considerations specific to AS should be considered. Bone loss is a well-recognized feature in AS. Osteoporosis is common and is largely related to disease activity, whereas vertebral fractures appear to be related to duration of disease and structural severity of the disease.61 The prevalence of vertebral fractures has been reported but variable.62 Cooper and colleagues reported an increased odds ratio of 7.7 (4.3 to 12.6).63 These fractures are often spontaneous, low-trauma fractures. Pregnancy can also lead to osteoporosis and/or bone density loss, which most often occurs in the lumbar spine in the peripartum period or within a few months after the birth of a child. Bone density loss related to pregnancy in combination with underlying bone density loss or loss of integrity due to AS may increase the risk of fracture even further and has been reported.64,65 This bone fragility should especially be considered during delivery when tremendous intrauterine pressure, most of which is transmitted to the perineum and lumbar spine, is created during active labor and pushing. If a patient has severe osteoporosis and/or poor spinal mobility, an elective cesarean section may be considered to reduce risks associated with active labor.
Ways to Improve Outcomes
The mechanical variants that result in patients with AS are important to consider during delivery. AS often results in inflammation of and/or fusion of the pubic symphysis or sacroiliac (SI) joints. Hormones released during pregnancy such as relaxin cause the symphysis pubis and SI joints to soften and separate to some degree to prepare for the birthing process. For example, the nonpregnant gap of the pubic symphysis is 4 to 5 mm but in every pregnancy there will be an increase in 2 to 3 mm. If fusion interferes in adequate separation, it can create a mechanical obstacle during labor and delivery. In some cases, this may cause increased pain and/or lead to need for cesarean section delivery as opposed to vaginal birth. As noted earlier, Ostensen and colleagues reported that, compared with healthy women, caesarean section was more frequently performed in patients with AS and in 58% of the cases AS was the indication for cesarean delivery.57 Aside from potential difficulties with labor mechanics, these ligament and/or joint changes in the setting of underlying inflammation may worsen pain symptoms and increase SI joint or pubic symphysis dysfunction (a disorder common in healthy pregnant women).
Vasculitis
Takayasu’s Arteritis
Takayasu’s arteritis, a large vessel vasculitis that leads to stenoses and aneurysms in the aorta and its branches, primarily affects young women. More than 150 pregnancies in women with Takayasu’s arteritis have been published, most coming from five case series.66–70 The primary complication seen in these pregnancies was hypertension (≤30% of pregnancies) with almost 20% developing preeclampsia.71 Serious maternal complications including myocardial infarction, aortic aneurysm and rupture, renal insufficiency, and pulmonary embolism have been reported, but the majority of women appear to do well without progression of Takayasu’s arteritis during pregnancy. More than 80% of pregnancies resulted in the delivery of a healthy baby, though preterm delivery, either spontaneous or induced for preeclampsia, was common. Intrauterine fetal demise was reported in 8% of pregnancies, and intrauterine growth restriction (a marker of poor placental perfusion) occurred in almost 20% of pregnancies.71
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree