CHAPTER 19 Partial-Thickness Rotator Cuff Tears
Our knowledge and understanding of partial-thickness rotator cuff tears (PTRCTs) have grown with the advent of shoulder arthroscopy and refinements in magnetic resonance imaging (MRI) techniques. Despite improvements in detection, the magnitude of pain and disability attributable to partial cuff tears remains uncertain and the natural history of these lesions is largely unknown. PTRCTs have shown a limited capacity for spontaneous healing and tend to progress over time.1 Considerations in the management of partial cuff tears include the patient’s age, activity preference, shoulder demands, response to nonsurgical treatment, location and extent of tearing, associated pathology, and surgeon’s level of skill and experience.
ANATOMY, PATHOANATOMY, AND BIOMECHANICS
A detailed understanding of rotator cuff anatomy will enable the clinician to appreciate and effectively manage the observed pathology. The tendons of the supraspinatus and infraspinatus blend together 15 mm medial to their insertion onto the greater tuberosity whereas the infraspinatus and teres minor coalesce closer to their musculotendinous junctions.2 As the individual rotator cuff tendons splay out and interdigitate, they form a common insertion onto the greater tuberosity. A superficial anterior extension of the supraspinatus contributes to a sheath that becomes the roof of the bicipital groove and a deep superior extension of the subscapularis helps form the floor.3 This latter relationship contributes to the anterior sling of the biceps and may help to explain, in part, the frequent coincident findings of a partial tear of the superior boarder of the subscapularis with anterior subluxation of the long head of the biceps. The coracohumeral ligament consists of a condensation of capsular tissue without a true ligamentous structure.4 This thick band originates on the base of the coracoid, reinforces the biceps sheath, and then blends with the capsule deep to the supraspinatus and infraspinatus.
On histologic evaluation, the rotator cuff was found to consist of five distinct lamina.2 Layer 1 extends from the base of the coracoid to the greater tuberosity and is composed of superficial fibers that overlie the cuff tendons. Layers 2 and 3 contain the supraspinatus and infraspinatus tendons. Layer 2 is composed of relatively large collagen fibers, which are parallel to the axis of the tendon. The fibers of layer 3 are smaller, more random, and form a meshlike network. Layer 4 constitutes the deep extension of the coracohumeral ligament, coursing from anterior to posterior, and layer 5 is comprised of the true capsule, which extends from the glenoid rim and labrum to the humeral neck. Differential strain in the decussating fibers of different tendon layers contributes to intratendinous delamination. The bursal layers have approximately double the ultimate tensile strength and are more elastic than those near the articular surface.5 The primary vascular supply for the rotator cuff derives from an anastomotic network between layers 2 and 3, with contributions from the suprascapular and subscapular arteries.6 Additional blood supply is via retinacular vessels from the humeral circumflex arteries that enter the tuberosities. The deeper articular side of the cuff contains smaller arterioles and is relatively hypovascular, particularly in the supraspinatus region.7
The region on the greater tuberosity where the rotator cuff inserts has been termed the footprint. This attachment site varies in breadth, averaging 12.7 mm for the supraspinatus, 13.4 mm for the infraspinatus, and 17.9 mm for the subscapularis.8 The distance of the tendinous insertion to the articular surface increases from approximately 1 mm near the anterior supraspinatus to 13.9 mm at the inferior aspect of the teres minor. The mean anteroposterior distance of the supraspinatus is 1.63 cm, the infraspinatus 1.64 cm, and the subscapularis 2.43 cm.9 Gross cadaveric dissections have helped identify a thick band of tissue termed the rotator cable, which courses from anterior to the biceps to the posterior aspect of the infraspinatus. Within this arc lies the rotator crescent, consisting of the distal portions of the supraspinatus and infraspinatus insertions.10 The cable is approximately 2.5 times thicker than the crescent that it surrounds. The cable appears functionally similar to a suspension bridge and stress-shields the crescent to some degree. The prevalence of this distinct cable-crescent anatomic pattern is uncertain, but appears to increase with age.
Biomechanical research has improved our understanding of rotator cuff dysfunction. It has been shown that the articular surface of the rotator cuff has an ultimate stress to failure approximately half that of the bursal surface,11 and that tear propagation often progresses from the articular to the bursal surface of the cuff. With extension of the tear, small defects have the capacity to develop into full-thickness tears. A cadaveric study was conducted to create three partial-thickness tear patterns—articular surface, bursal surface, and midsubstance.12 Finite element analysis demonstrated that in all three types of tears, as tension was placed on the cuff muscles, a high stress concentration appeared at the articular surface and at the site of the tear. In a separate cadaveric investigation, tears of 25%, 50%, and 75% thickness were created and strain was measured at 45, 60, and 90 degrees of abduction.13 Articular-sided tendon strain increased consistently across the supraspinatus tendon with tear depths of 50% and 75%, but not 25%. Repair returned the supraspinatus strain almost to the intact state.
These findings lend biomechanical support for the clinical practice of repairing partial tears equal to or greater than 50%. Joint position and its relation to articular-sided tears has also been studied in a cadaveric model. An MRI-based technique to was used quantify cuff strain in shoulder positions of 15, 30, 45, and 60 degrees glenohumeral abduction in the scapular plane.14 Intratendinous strain was seen to increase significantly across all tendon regions with increasing abduction. These findings are particularly relevant to overhead athletes and workers with repetitive overhead demands.
CAUSES, INCIDENCE, AND CLASSIFICATION
Although the final common pathology for a partial-thickness rotator cuff tear is tendon fiber failure, the causes are mulifactorial.15 Both intrinsic and extrinsic causes have been hypothesized. Intrinsic causes first proposed by Codman and more recently advocated by Nirschl include age-related metabolic and vascular changes, including decreased cellularity, thinning of collagen fascicles, accumulation of granulation tissue, and dystrophic calcification. Those alterations may lead to degenerative tearing whereas shear stresses may contribute to the development of intratendinous lesions, including delamination. As degenerative changes occur, the ability of the rotator cuff to depress the humeral head during elevation may be significantly compromised. Consequently, superior migration and secondary wear and fraying on the bursal surface of the involved tendons may occur because of abutment on the inferior surface of the acromion.
The prevalence of rotator cuff disease is strongly associated with age, with the peak incidence of PTRCTs appearing to occur in the fifth and sixth decades of life. Many tears are minimally or asymptomatic and patients are not motivated to seek medical evaluation to detect them. Even a partial tear identified at diagnostic arthroscopy may be an incidental finding and not necessarily related to the patient’s primary symptoms. Of the partial-thickness tears that are symptomatic and create dysfunction, the supraspinatus is the most commonly involved. In a cadaveric study of 306 shoulders, 32% were found to have partial-thickness cuff tears and 19% full-thickness tears of the supraspinatus.16 Clinically, articular-sided tears are identified up to three times more frequently than those originating on the bursal surface. Intratendinous tears can be particularly difficult to detect. In one study of 249 cadavers in which the specimens were noted to be older, 13% had PTRCTs (18% bursal, 27% articular, 55% intratendinous).17 In a separate evaluation of 249 cadaveric specimens, intratendinous tears were the most common type of partial-thickness tears (7.2%), followed by articular-sided tears (3.6%) and bursal-sided tears (2.4%).18 Partial tears of the subscapularis tendon also occur. In a study of 46 cadaveric shoulders, 17 were found to have partial articular-sided tears of the subscapularis near the superior boarder.19 Of those tears, 30.4% also had a lesion of the long head of the biceps, suggesting a significant association.
Useful classifications of partial-thickness rotator cuff tears were lacking prior to the publication of Ellman’s scheme which included location (articular, bursal, intratendinous), depth (grade 1 < 3 mm deep; grade 2, 3 to 6 mm deep; and grade 3 > 6 mm deep), and area (in mm2).20 Snyder offered a somewhat more geographic and qualitative classification, including location (articular, bursal, complete), and tear severity (0 = normal cuff; 1 = synovial irritation < 1cm; 2 = superficial fraying or failure of cuff fibers < 2 cm; 3 = substantial tearing of cuff fibers < 3 cm; 4 = severe partial cuff tearing or flap > 3 cm).21 Intratendinous tears have been difficult to accurately classify, in part because of the difficulty in defining the extent arthroscopically. To date, most estimates of the tear depth have relied on the extent of the footprint exposed after débridement of the damaged tissue from the greater tuberosity.9 An instrument of known dimension, (e.g., a 5.5-mm shaver) is used to measure the breadth of the region on the tuberosity devoid of normal fiber attachment. Calibrated probes have also been developed in an attempt to improve the accuracy in measuring tear thickness. A study designed to assess the preoperative interobserver grading in the classification of PTRCTs has shown poor agreement in predicting the depth of a partial-thickness tear.22 Our current methods to grade partial cuff tears likely provide only a rough estimate.
DIAGNOSTIC IMAGING
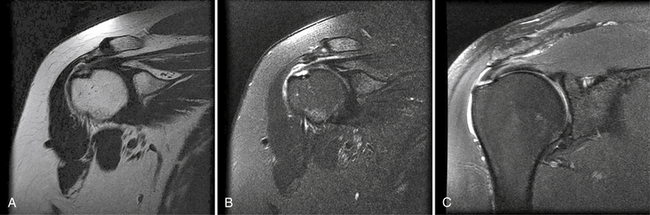
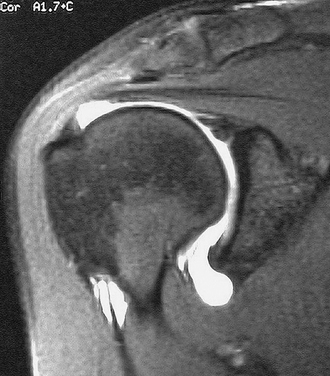
In the past, MRI demonstrated only moderate success in identifying partial-thickness tears of the rotator cuff. Enhancements to MRI evaluation including sequence manipulation (fat suppression), contrast arthrography, and differential arm positioning, have improved the accuracy of evaluating partial-thickness cuff tears. Of added value is the ability of MRI to identify associated pathology, including biceps and labral disorders. The presence of a PTRCT is suggested by an increased signal in the rotator cuff on T1-weighted images (without evidence for loss of continuity; Fig. 19-1A) combined with a focal defect on T2-weighted images that is intratendinous or limited to the articular or bursal surface (see Fig. 19-1B and C).23,24 It may be difficult to differentiate a high-grade partial-thickness tear from a small full-thickness tear. The accuracy in identifying articular surface and delaminating tears is improved with the addition of intra-articular contrast (Fig. 19-2). In a review of 76 patients who underwent MR arthrography (MRA) and subsequent arthroscopy, 26 of 28 patients with a partial articular-sided cuff tear were accurately identified preoperatively.25 Sensitivity was 84%, with a positive predictive value of 93%. A specificity of 96% and overall accuracy of 91% (69 of 76) were reported. In another evaluation of 50 patients in which MRA and arthroscopy were again correlated, the sensitivity and specificity of MRA were 91% and 85%, respectively, with a positive predictive value of 84% and a false-negative rate of 9%.26 MRA with the patient’s shoulder placed in the abduction, external rotation (ABER) position improves the sensitivity for depicting PTRCT,27 particularly those in whom a horizontal or delaminating component is present.28 In that position, tension within the posterior cuff is decreased and the contrast can more readily infiltrate an articular-sided cuff defect. For most patients with suspected partial-thickness cuff tears, especially young, overhead throwing athletes, MRA is the imaging modality of choice.