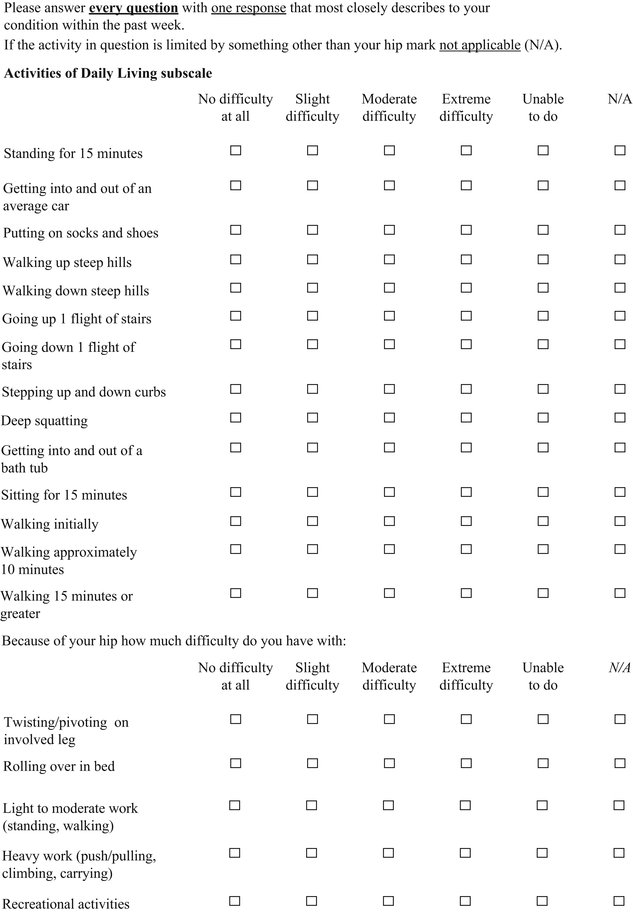
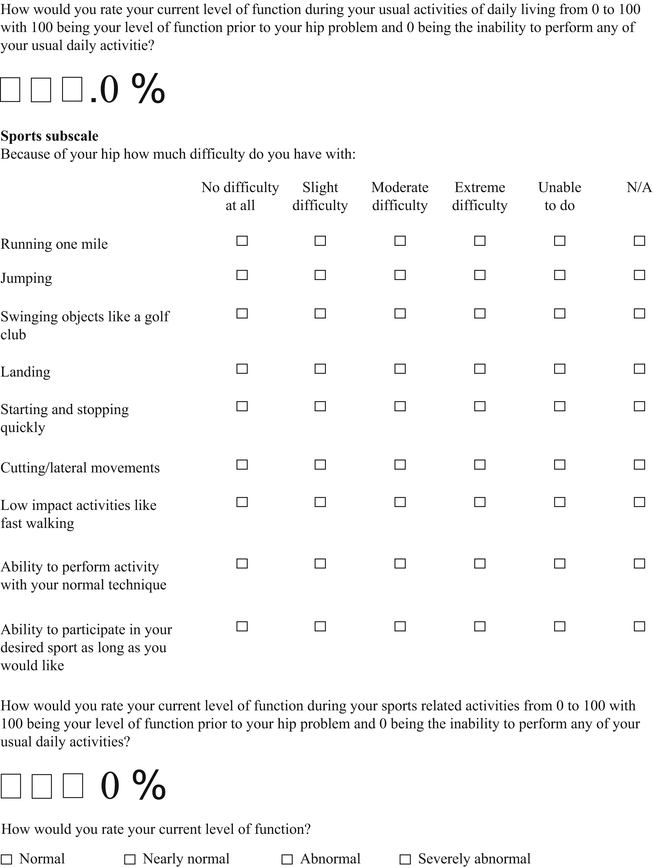
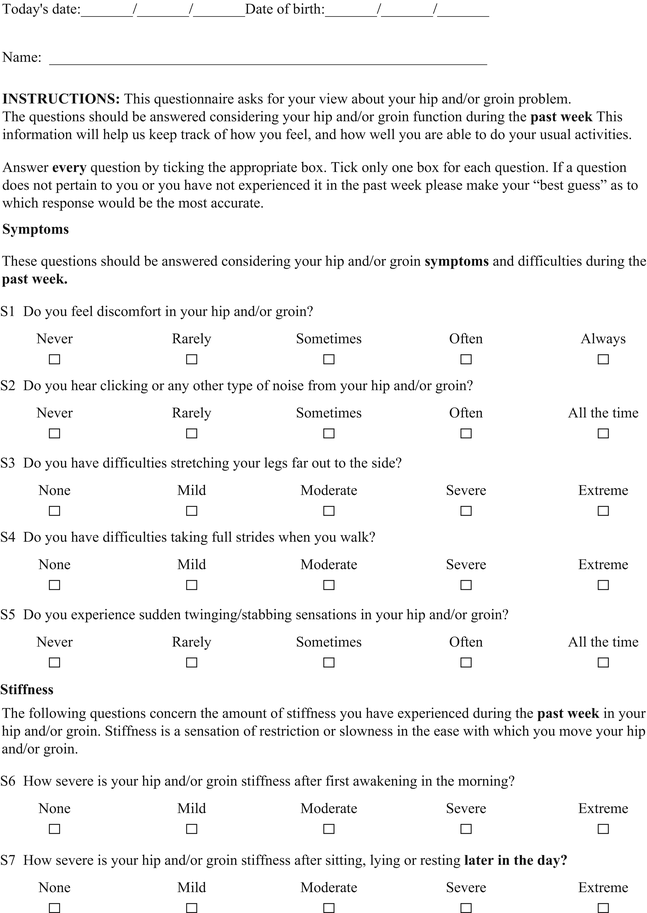
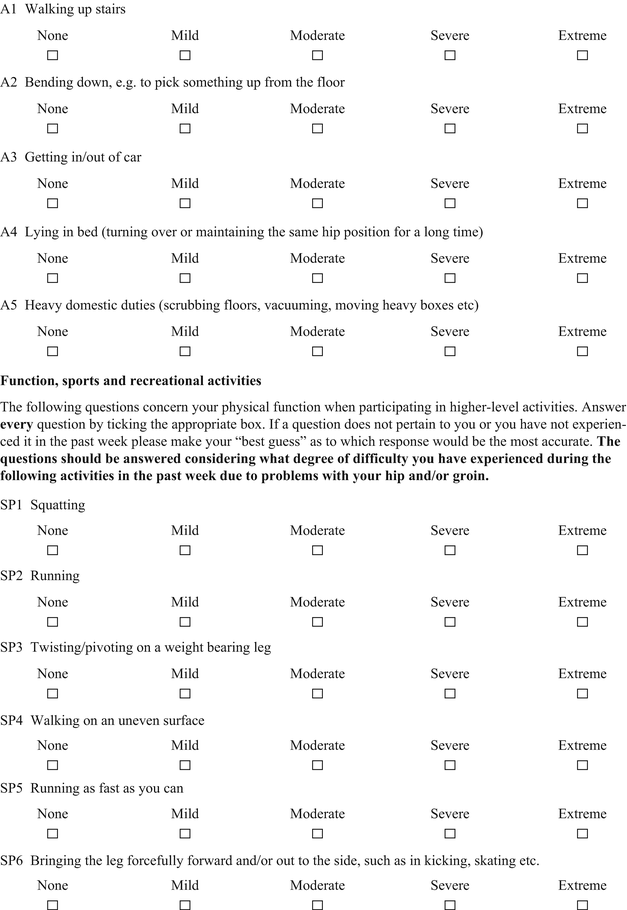
Hip Outcome Score (HOS)
The Hip Outcome Score (HOS) was specifically developed for younger more active patients between the ages of 13 and 66 years [22]. The HOS is a patient-administered tool (Table 2) that was designed to assess self-reported functional status; therefore, symptoms were not considered part of the functional assessment. The HOS includes two subscales: activities of daily living (ADL) and sports. Items were generated by physicians and physical therapists and reduced by factor analysis. No patients were involved with item generation. The tool does show internal consistency, as determined by Cronbach’s coefficients. True test-retest reliability was not measured since all patients had an intervention (i.e., arthroscopic surgery) between administrations at baseline and an average of 7 months follow-up [23]. The HOS demonstrated good construct validity, as measured by convergent and divergent validity with the Short Form 36 questionnaire using Pearson correlation coefficients. The items in both subscales were shown to be responsive. By design the HOS does not represent a true patient-derived outcome since it does not include items of specific concern to patients such as symptoms and work-related, social, or emotional issues. It should be considered a well-designed and evaluated functional outcome measure. The scoring of the HOS is somewhat complicated since each subscale is scored separately as a percentage score. The ADL subscale has 19 items but only 17 are scored. The items pertaining to sitting and putting on socks and shoes are not included. The sports subscale has 9 items. Each item on both scales is scored from 4 to 0, with 4 indicating “no difficulty” and 0 indicating “unable to do.” There is a “not applicable” option as well. The percentage score is calculated by comparing the item total score divided by the highest potential score multiplied by 100.
Copenhagen Hip and Groin Outcome Score (HAGOS) and International Hip Outcome Tool (iHOT)
More recently two newer patient-reported outcomes have been developed: the Copenhagen Hip and Groin Outcome Score (HAGOS) [12] and the International Hip Outcome Tool (iHOT) [11]. The HAGOS (Table 3) has been developed using standardized format by identifying a specific population of interest, generating items, item reduction, and determination of validity, reliability, and responsiveness. This instrument has the goal of evaluating “hip and/or groin disability related to impairment (body structure and function), activity (activity limitations) and participation (participation restrictions) according to the International Classification of Functioning, disability and health (ICF), in young to middle-aged physically active patients with hip and/or groin pain.” The item generation process was determined as a result of a systematic review of the literature. The authors chose to include items from the HOOS and HOS questionnaires [22–24]. Forty-three questions in total (40 from the HOOS and 3 from the HOS) formed the basis of the HAGOS. An expert group, including two orthopaedic surgeons, one physician, and four physiotherapists, added an additional 8 questions. A representative focus group of 20 patients added 2 and removed one question, resulting in a 52-item questionnaire. The item reduction process involved 101 patients. A combination of the frequency and importance of each question to these patients, as well as reliability testing, was used to determine which items should be included. Fourteen items were subsequently removed as a result of further consensus among the authors. One final item was removed as a result of factor analysis resulting in a final questionnaire of 37 questions in six separate subscales: Pain (10 items), Symptoms (7 items), ADL (5 items), Sport/Rec (8 items), PA (2 items), and QOL (5 items) [33]. Content validity was considered due to the patient (N = 25) and expert group (N = 7) involvement. The questionnaire includes items that are related to soft tissue injury and clearly is distinct from the other questionnaires in this respect. Test-retest reliability was measured 1–3 weeks after baseline in 44 out of the 101 patients and deemed to be very high in all subscales, with intra-class correlation coefficients ranging from 0.82 to 0.91. The authors measured responsiveness at 4 months from baseline in 87 of the 101 patients. They compared the change scores to asking the patients on a 7-point Global Perceived Effect (GPE) score. The correlations with the HAGOS in each subscale were higher than hypothesized. They also measured the standardized response mean (SRM) and effect sizes (ES) on each subscale, which were noticeably higher in patients who had stated that they were “much better” and “better” on their GPE scores. The SRM and ES calculations ranged from 0.90 and 0.77, respectively, for the ADL subscale and 1.46 and 1.78 for the QOL subscale. Construct validity was determined by comparing the HAGOS to the SF-36, which has significant limitations. The SF-36 is a generic outcome measure with likely little relevance to the population at hand. Therefore it is not surprising that the comparison with respect to a priori correlations was satisfactory but not consistent. Finally, PROs should be able to measure the minimal important change (MIC) and/or minimal important difference (MID). The HAGOS showed that the MIC for each subscale ranged from 10 to 15 points based on using the estimate of one half of the reported standard deviation. The authors identified this limitation. If the HAGOS was used as the primary outcome measure in a clinical trial, more patients would be needed in order to achieve a meaningful sample size [12].
The latest PRO advocated for patients with hip disorders is the International Hip Outcome Tool (iHOT) [11] . This PRO has previously been called the Hip Quality-of-Life Questionnaire and the MAHORN Hip Outcome Tool (MHOT). Developed with the cooperation of the Multicenter Arthroscopy of the Hip Outcomes Research Network (MAHORN), this tool was designed to address the outcomes of treatment in young active patients with hip disorders. This outcome measure included patients from the USA, Canada, England, and Switzerland. This outcome measure was developed for active patients (18–60 years old; Tegner activity scale ≥ 4) presenting with a variety of hip conditions. This multicenter study recruited patients from the practices of a group of international hip arthroscopy and arthroplasty surgeons. The outcome was created using a process of item generation (51 patients, 4 orthopaedic surgeons, and 4 physiotherapists), item reduction (150 patients), and pretesting (31patients). The questionnaire was tested for test-retest reliability (123 patients), face, content and construct validity (51 patients), and responsiveness over a 6-month period in post-arthroscopy patients (27 patients) for a total of 433 patients. Initially, 146 items identified through patient query were reduced to 60 through item reduction and categorized into four domains – (a) Symptoms and Functional Limitations, (b) Sport and Recreational Physical Activities, (c) Job-Related Concerns, and (d) Social, Emotional, and Lifestyle Concerns – and formatted using a visual analogue scale. Pretesting confirmed appropriate wording, content, and formatting. Test-retest reliability showed Pearson correlations greater than 0.80 for 33 of the 60 questions. These 33 questions were formulated into a self-administered questionnaire using a visual analogue scale response format from 0 to 100. A score of “0” reflects the worst possible quality of life and “100” the best possible score. Intra-class correlation statistic as a reflection of reliability was 0.78, and Cronbach’s alpha as a reflection of internal consistency was 0.99. Face and content validity were ensured because of the extensive involvement of patients, the expert developers, and the MAHORN group. Construct validity was demonstrated with a correlation of 0.81 to the Non-arthritic Hip Score. Responsiveness was demonstrated with a paired t-test (p ≤ 0.01), effect size was 1.95, standardized response mean was 1.69, and the responsiveness ratio was 6.7. The calculated minimal clinical important difference (MCID) was 6 points out of the total 100-point scale. These properties make the iHOT very attractive as an outcome tool, since the MCID can be used interchangeably with the MIC, in calculating sample sizes for prospective research studies. As a result this highly validated, truly patient-based, and responsive questionnaire has been recommended for use in randomized clinical trials and prospective cohort studies [11]. An ongoing effort by the MAHORN group continued to develop a 12-item questionnaire, i.e., the iHOT-12 (Table 4), that reflects the longer version, the iHOT-33 [10] (Table 5). The IHOT-12 utilizes 12 of the same questions with similar properties, includes all four domains, and has been recommended for clinical use rather than for research purposes. In this way, busy clinicians with minimal resources can measure a PRO with their patients. The IHOT-12 should be simple enough for phone administration and paper- or computer-based formats and will minimize responder burden for the patients [10].
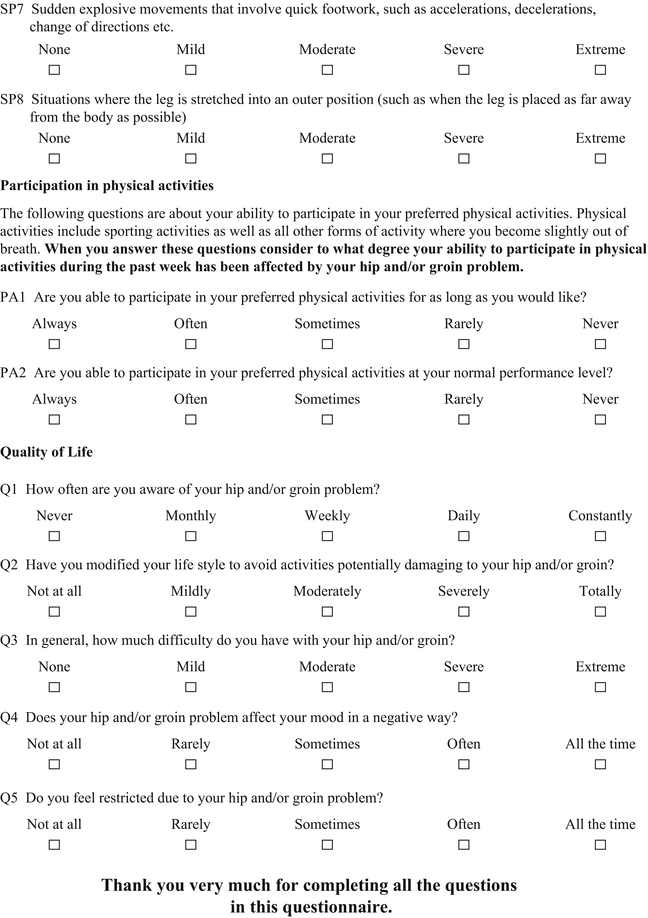
Table 4




iHOT-12. Reprinted from [10], with permission from Elsevier
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree