103 Osteonecrosis
Corticosteroids constitute the most common cause of nontraumatic osteonecrosis.
The femoral head is the most common site of osteonecrosis.
Bisphosphonate use is associated with osteonecrosis of the jaw.
Nonsurgical treatment of osteonecrosis does not change the natural history of the disease.
Due to the lack of successful treatment options, new modes focus on prevention of osteonecrosis.
Osteonecrosis literally means “bone death” (ossis [Latin] = bone; necrosis = killing or causing to die). Other synonyms include avascular necrosis, ischemic necrosis of bone, aseptic necrosis, and subchondral avascular necrosis. The term osteonecrosis dissecans is sometimes used synonymously with osteonecrosis, although, strictly speaking, it is a consequence of osteonecrosis involving dessication of bone leading to fracturing or cracking of bone. The concept of bone death was first described by Hippocrates,1 but the first clinical description of osteonecrosis was a case of sepsis-induced bone death described by Russell in 1794.2 It was almost a century later that bone death was described to occur in the absence of infection.3 The first report of osteonecrosis in a deep sea diver appeared in 1936.4 The pathogenesis of osteonecrosis is complex, but whatever the mechanism, bone death ultimately occurs as a result of complete or partial disruption of the delivery of oxygen and/or nutrients to the bone and surrounding tissues. It is likely that multiple molecular mechanisms may be simultaneously in play in order for osteonecrosis to occur.5,6
Epidemiology
The prevalence of osteonecrosis is unknown, but it is estimated that there are 10,000 to 20,000 new patients diagnosed per year in the United States. Osteonecrosis occurs in 15% to 80% of patients with femoral neck fractures.7 Ten percent of the 500,000 hip replacements done in the United States each year are thought to be for osteonecrosis.8 The disease primarily affects men, with a notable exception for osteonecrosis associated with systemic lupus erythematosus, which has a significant female predominance. Osteonecrosis primarily occurs in the third to fifth decade of life.9 As a result of this age distribution, long-term morbidity can be significant because most hip replacements have a finite period of viability.
Etiology
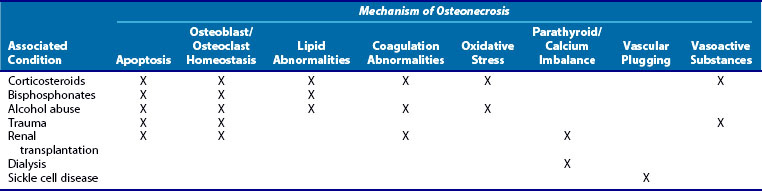
Osteonecrosis has been linked to numerous conditions (Table 103-1). The strength of a causal relationship varies greatly, and in some cases only case reports have been published. The most common cause of nontraumatic osteonecrosis is corticosteroid use, which was first described in 1957.10 Although other adverse effects of corticosteroids are perhaps better known, osteonecrosis of the femoral head is one of the serious complications.
Table 103-1 Conditions Associated with Osteonecrosis
| Dietary, Drugs, and Environmental Factors |
| Musculoskeletal Conditions: Compromise in Structural Integrity |
| Metabolic Diseases: Abnormality in Fat or Other Metabolic Component |
| Hematologic Conditions: Abnormalities in Blood Components |
| Rheumatologic Conditions |
| Infectious Diseases |
| Oncologic Disorders, Transplantation, and Their Treatment |
Studies have attempted to determine the duration of use and the dosages of corticosteroids necessary to precipitate osteonecrosis. There are several forms of corticosteroids of differing potency and half-life, and dosages and duration of use vary between studies, so any conclusions about a “safe” dose of corticosteroids are wrought with potential confounding variables and errors. In one study of 20 patients diagnosed with stage 1 osteonecrosis by magnetic resonance imaging (MRI), the interval between the use of steroids and diagnosis ranged from 1 to 16 months.11 The cumulative dose of steroids in this study ranged from 1800 to 15,505 mg (mean, 5928 mg) of prednisolone or the equivalent. In other studies cumulative doses of steroids associated with osteonecrosis ranged from 48012 to 432013 mg of dexamethasone dose equivalence. A recent paper by Powell and colleagues14 attempted to collectively analyze the available literature to derive maximum safe levels for duration, maximum daily dose, and average daily dose of corticosteroids. The study confirmed that many other confounding variables affect the development of osteonecrosis, making analysis of dose-response risk for an isolated association difficult. Nonetheless, corticosteroid-induced osteonecrosis is dependent on dosage and the risk factor is higher with the long-acting steroids and with parenteral usage.
Additional host-inherent risk factors also play a role in susceptibility. The incidence of osteonecrosis in a group of patients receiving glucocorticoid replacement therapy for primary or secondary adrenal insufficiency was 2.4%. In a study of renal transplantation patients, the 26 patients who developed osteonecrosis had a higher cumulative oral dose of prednisone after 1 and 3 months compared with 28 control transplant patients who did not develop osteonecrosis.15 A separate study estimated the incidence of osteonecrosis in renal transplant patients to be 5%.16 There is no evidence to consistently link the use of topical, inhaled, or nasal corticosteroids to osteonecrosis. The evidence for an association between osteonecrosis and intramuscular or intra-articular corticosteroids is limited to case reports.17 Parenteral use poses a higher risk because of rapid absorption and longer half-life of the drugs used.
Bisphosphonate-induced osteonecrosis of the jaw is particularly interesting because of the intended use of bisphosphonates on bone diseases.18–20 There has been a link between cigarette smoking and osteonecrosis, with smokers having a threefold higher relative risk for developing osteonecrosis, independent of all other factors.21,22
The association between osteonecrosis and alcohol consumption was first described in 1922.23 A study of patients with idiopathic osteonecrosis revealed that the risk of osteonecrosis increased with increasing daily consumption of alcohol.21 The subjects were divided into three groups on the basis of their alcohol consumption of less than 400 mL/week, 400 to 1000 mL/week, and greater than 1000 mL/week, and the relative risk of osteonecrosis, independent of corticosteroid use or smoking, was 3-fold, 10-fold, and 18-fold, respectively, when compared with hospital controls. Liver damage was also found unnecessary for the development of osteonecrosis in alcohol-consuming patients, although elevated liver enzymes may be present.24 The incidence of osteonecrosis in patients who received treatment for alcoholism was 5.3%. The femoral head was again the most common site (82 of 92 lesions), with the other 10 sites involving the humeral head.25
Musculoskeletal conditions can lead to osteonecrosis in children. Legg-Calvé-Perthes disease was first described in children between 3 and 12 years of age in 1910.26–28 Femoral head osteonecrosis is a feature of this disease and has been linked to trauma,29,30 congenital hip dislocation,31 and transient synovitis.32 Bilateral involvement is common, and associated clinical manifestations include abnormal growth and stature,33,34 delayed skeletal maturation,35 disproportionate skeletal growth,33 congenital anomalies,36 and abnormal hormone levels.37,38 Children with acute lymphoblastic leukemia can develop osteonecrosis39,40 as well, but this may be a result of steroid use. An additional risk factor for this cohort of patients is high body mass index.41
Osteonecrosis has also been associated with metabolic disorders and in pregnancy. Diagnosis is often delayed until months after delivery. Women who develop ostenecrosis in pregnancy tended to have a small body frame and a large weight gain.42
Hematologic conditions have been associated with osteonecrosis. The long-term morbidity of osteonecrosis in patients with sickle cell anemia is dismal.43 Common deformities include decreased mobility, abnormal gait, and leg-length discrepancy.44 Osteonecrosis in hemophilia patients has been reported, but no statistically reliable causal link can be established.45–50
Dysbaric osteonecrosis was first described in construction workers in the Elhe tunnel exposed to high-pressure environments.51 The prevalence of dysbaric osteonecrosis is 4.2% in divers and 17% in compressed air workers.52 Patients with dysbaric osteonecrosis may have more than one lesion, and common sites besides the femoral head include the tibia and the humeral head and shaft. The condition is not related to decompression sickness, and although proper decompression procedures can reduce “the bends,” they do not have any effect on the development of osteonecrosis, which can occur months or years after the last exposure to high-pressure environments.
Osteonecrosis has also been associated with a number of infectious diseases including severe acute respiratory syndrome (SARS). Many patients who contracted SARS in the early 2000s received treatment with corticosteroids, and some subsequently developed osteonecrosis.53 The incidence of osteonecrosis appears higher in this group of patients compared with patients with other conditions who were treated with corticosteroids.54 Chan and colleagues55 reported five children with SARS treated with corticosteroids who developed osteonecrosis.
Clinical Features
In addition to the femoral head, osteonecrosis can affect other sites including the humeral head,56–59 femoral condyles60–63 and proximal tibiae,61,64–66 wrists and ankles,67 bones of the hands and feet,68 the vertebrae,69–71 jaw,72–75 and bony structures of the face.76 Osteonecrosis of the humeral head is the second most commonly seen location, and pain is usually in the shoulder and associated with reduced range of motion and weakness. Pain in the ankle is the main presenting symptom in nontraumatic osteonecrosis of the talus, and in some cases, the disease had already progressed to Ficat and Arlet stage 3 by the time of presentation of pain.67 Kienböck’s disease involves osteonecrosis of the lunate. Patients present with pain in the radiolunate joint, along with weakness and limitation of motion. Keinböck’s disease appears to be related to manual labor. Soccer players have been reported to develop osteonecrosis of the foot,77 and football players may be prone to developing osteonecrosis of the hip.78
Table 103-2 shows the Modified Steinberg system for staging osteonecrosis. The Association of Research Circulation Osseous (ARCO) has proposed a modification to the Ficat and Arlet system, adding a stage 0 or patients with negative imaging studies but who are at risk for developing osteonecrosis. In addition, stages 1 and 3 are further stratified to take into account lesion size, location, and extent of collapse.79 In 2001 the Japanese Ministry of Health, Labor and Welfare proposed revising criteria for the diagnosis and staging of osteonecrosis of the femoral head.80 Diagnostic criteria included the following: (1) collapse of the femoral head without joint space narrowing or acetabular abnormality on plain radiograph, (2) demarcating sclerosis in the femoral head without joint space narrowing or acetabular abnormality, (3) “cold in hot” on bone scans, (4) low-intensity band on T1-weighted MRI, and (5) trabecular and marrow necrosis on histology. If a patient fulfills two of the five criteria, the diagnosis is established. The working group also proposed four types of lesions on the basis of extensiveness and defined stages of disease on the basis of diagnostic imaging.
Table 103-2 Modified Steinberg Staging Systems for Osteonecrosis
| Stage | Radiographic Appearance | Reversible |
|---|---|---|
| I | Normal radiographs, but abnormal bone scan or magnetic resonance image | Yes |
| II | Lucent and sclerotic changes | Yes |
| III | Subchondral fracture without flattening | No |
| IV | Subchondral fracture with flattening or segmental depression of femoral head | No |
| V | Joint space narrowing or acetabular changes | No |
| VI | Advanced degenerative changes | No |
Bone Marrow Edema
A specific syndrome known as bone marrow edema syndrome has been described and was initially thought to be a precursor to osteonecrosis, but it is now believed to be a separate entity. Bone marrow edema is a transitory, self-limiting condition typically seen in middle-aged men and in women in their third trimester of pregnancy. Patients complain of pain, limited range of motion, and an abnormal gait. Osteopenia is detected on conventional radiographs, and MRI confirms this with a low signal on T1-weighted images and a high signal on T2-weighted images. The three phases of bone marrow edema syndrome include an initial phase lasting about 1 month, followed by a plateau phase lasting 1 or 2 months, and finally a regression phase lasting for an additional 4 to 6 months.81 Subchondral fractures do not occur. Biopsy specimens obtained in the initial phase show diffuse interstitial edema, fragmentation of fatty marrow cells, and increased new bone formation.82
A study of 24 cases of bone marrow edema syndrome of the knee showed that although migrating bone marrow edema occurred in a third of patients at a 5-year follow-up, the patients were asymptomatic and MRI signal alterations had resolved. Biopsy specimens of affected bone were obtained using arthroscopic surgery and core decompression, and histology revealed areas of bone marrow edema and vital trabeculae covered by osteblasts and osteoid seams. None of the cases progressed to osteonecrosis.83
Pathogenesis
Anatomic Considerations in Trauma-Related Osteonecrosis
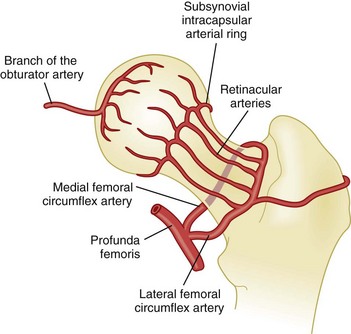
Some of these anatomic features may render the femoral head particularly vulnerable to ischemia. The retinacular arteries are believed to supply 80% of the femoral epiphysis. Compromising this critical vascular system may lead to osteonecrosis originating in the anterosuperior aspect of the femoral head, as indicated by angiographic studies in early osteonecrosis in which these arteries are not visualized. A schematic of the blood supply to the femoral head is shown in Figure 103-1.
Histologically, after an infarct, a rim of bony thickening or sclerosis begins to form at the margins of the infarcted area. If the necrotic lesion is within the weight-bearing region of the femoral head, subchondral fractures follow. With repeated microfractures and continued weight bearing, the original fracture cannot heal completely and new fractures appear. The secondary fracture propagates along the junction between subchondral bone and the necrotic segment. As time goes on, the femoral head becomes flattened and eventually collapses. A nonspherical head articulating with the acetabulum produces friction and erosion and loss of cartilage. The cycle repeats itself, and the structure of the joint deteriorates, leading to degenerative changes and eventual total joint destruction.84
Nontraumatic Osteonecrosis
The immunologic changes occurring in nontraumatic osteonecrosis may help explain why corticosteroids are particularly dangerous to the integrity of the blood supply of the femoral hip. Some have likened osteonecrosis to “coronary disease” of the hip85,86 and propose that the same mechanisms that cause ischemia of the myocardium may also cause ischemia of the femoral head (Table 103-3).
Mechanical and Vascular Considerations
In Legg-Calvé-Perthes disease, obstruction to venous drainage elevates intraosseous pressure and consequently elevates intra-articular pressures. In a study of patients with Legg-Calvé-Perthes disease, bone scintigraphy using Tc99m methylene diphosphonate (Tc99m MDP) was employed to measure arterial and venous flow in the diseased hip. Although arterial flow was normal, there was significant disruption in venous drainage.87 This disturbance was reproduced in a dog model in which injection of silicone was used to obstruct venous flow distal to the hip.88 Ischemia resulted from the obstruction to venous drainage, leading to a cessation of endochondral ossification in the preosseous ephiphyseal cartilage and the physeal plate. Widening of the joint space ensued, followed by revascularization of the epiphysis and deposition of new immature bone. A weakened or unstable femoral epiphyseal plate resulted, and the subchondral bone became prone to segmental collapse and fracture.89
The increased vulnerability of bone to compression disorders has been explained by several factors including the relative rigidity of bone and inability to absorb increased gas pressure, inherent poor vascularization, and gas supersaturation of fatty marrow.90 A sheep model of dysbaric osteonecrosis has been developed. Exposure to compressed air at pressures of 2.6 to 2.9 atmospheres for 24 hours results in extensive bone and marrow necrosis. The authors proposed that the initial event involving elevated intramedullary pressures leads to the formation of nitrogen gas bubbles in the fatty marrow of the long bones. Radiography shows medullary opacities and endosteal thickening. Later, neovascularization of previously ischemic fatty marrow occurs, followed by new bone formation. Osteonecrosis occurs in subchondral cortical bone with marrow fibrosis and osteocyte loss.91
Changes in the vasculature, through injury or inflammation from other diseases, may in turn lead to a compromise in blood flow. Examples include structural damage to arteriolar walls, degeneration of the tunica media, smooth muscle cell necrosis, and disruption of the internal elastic lamina. These changes can lead to eventual hemorrhagic infarction, which was observed in a study of 24 core biopsy specimens from osteonecrotic femoral heads. The changes did not occur in 11 femoral heads with osteoarthrosis.92
Osteoimmunology
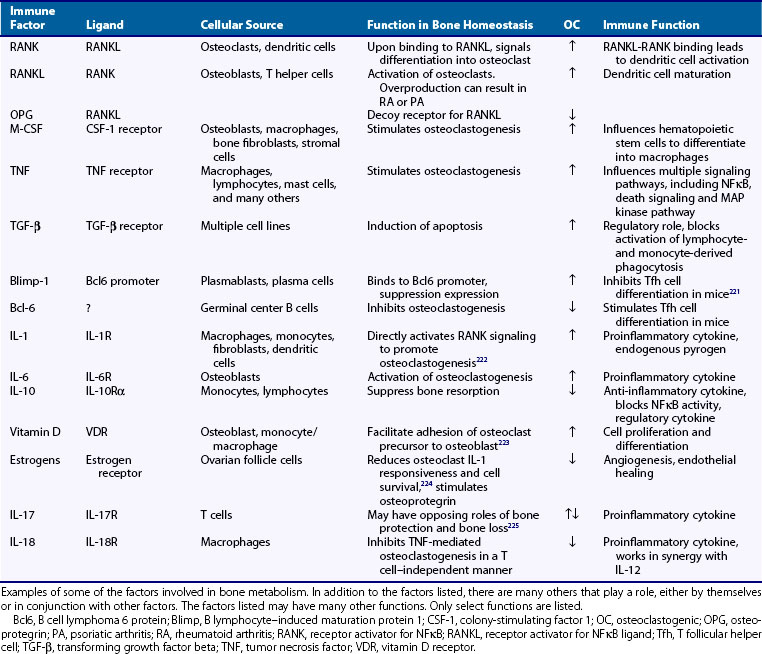
Immune factors involved in bone homeostasis include receptor activator of NFκB (RANK) and its ligand (RANKL), IL-1, IL-6, IL-10, TFG-β, TNF, CD80, CD86, CD40, macrophage colony-stimulating factor (M-CSF), NFATc, and vitamin D. (See Table 103-4 for roles and function.) Many of these factors can be categorized into one of two categories, those with the overall effect of inducing osteoclastogenesis and those that inhibit osteoclastogenesis. In addition, factors involved in cell survival and apoptosis such as Blimp-1 and Bcl6 may also play a role. RANKL is expressed on osteoblasts and is critical for the differentiation and proliferation of osteoclasts. Because transcription of factors involved in the regulation of bone homeostasis is often influenced by glucocorticoids, this may begin to explain why steroids may be associated with osteonecrosis.
Osteoblast/Osteoclast Balance
Any disturbance in the normal homeostasis between bone deposition and bone resorption can lead to bone disease. Moreover, defective bone deposition or bone resorption in which new bone is formed in an aberrant manner can lead to disease. Alcohol can affect the ability of mesenchymal stem cells to differentiate into osteogenic lineages. The bone marrow in the proximal head of femurs was isolated during hip replacement surgery from 33 patients with either femoral neck fractures or alcohol-induced osteonecrosis. The cells from femurs of patients with alcohol-induced osteonecrosis showed a reduced ability to differentiate into osteoblasts.93 A subsequent study compared the mesenchymal stem cells from patients with hip osteoarthritis, idiopathic osteonecrosis, and nontraumatic osteonecrosis associated with steroid or alcohol use. In idiopathic and alcohol-induced osteonecrosis, the ability of mesenchymal stem cells to differentiate into osteoblasts was decreased, but in steroid-induced osteonecrosis, it was elevated, although not to a statistically significant level. The adipogenic differentiation ability was similar in all four groups.94
Alterations in osteoblast function may also contribute to the pathogenesis of osteonecrosis. In one study, osteoblastic cells were obtained from bone biopsy specimens from the intertrochanteric region of the femur and of the iliac crest of 13 patients with osteonecrosis and 8 patients with hip osteoarthritis. Cell replication was measured on the basis of proliferation rate in secondary culture. Levels of alkaline phosphatase activity, collagen synthesis, and the sensitivity to 1,25-dihydroxyvitamin D3 were measured. The results indicated that although differentiation was not affected, the proliferation rate of osteoblastic cells was reduced in samples obtained from the patients with osteonecrosis compared with patients with osteoarthritic hips.95
Apoptosis and Osteonecrosis
Glucocorticoids can also act via its action on apoptosis of immune and bone cells. When mice were administered prednisolone for 27 days, increased metaphyseal apoptotic activity of both osteoblasts and osteoclasts were noted.96 The result was decreased bone turnover, density, and formation; increased formation of cancellous bone; and decreased trabecular width. The decreased bone turnover can be explained by the reduced osteoclast survival, and the reduction in trabecular width can be explained by a decrease in osteoblasts. An accumulation of apoptotic elements was also found in the region of the “fracture crescent” in the femurs of glucocorticoid-treated patients. On the other hand, glucocorticoids may also increase osteoclast survival, leading to increased bone loss. Clearly, the effect of osteoclast survival on bone disease is more complicated than at first glance, and it involves the interaction of the osteoclast with the osteoblast. Because osteoblasts are also responsible for osteoclast differentiation under the right circumstances, there exists a significant feedback system that maintains bone homeostasis.
Osteocyte death is also a feature of osteonecrosis. In a rat model, ischemia caused an induction in the expression of stress proteins, oxygen-regulated protein (ORP150) and hemoxygenase 1 (HO1). Induction of ischemia in these rates caused DNA fragmentation and the presence of apoptotic bodies in chodrocytes, bone marrow cells, and osteocytes.97 Both alcohol and corticosteroids can induce osteocyte apoptosis, possibly via lipid abnormalities.
Lipids and Osteonecrosis
In corticosteroid-induced osteonecrosis, the alteration in lipid metabolism parallels that of alcohol-induced osteonecrosis. In both cases, fatty infiltration of osteocytes has been postulated to occur.98–100 Table 103-5 lists lipid-altering effects of corticosteroids and alcohol. In addition, interosseous venous stasis affects the interosseous microcirculation, which can lead to hemodynamic and structural changes in the femoral head. The resulting decrease in blood flow leads to osteonecrosis. In chickens treated with steroids, fatty infiltration of the liver and fat cell hypertrophy and proliferation in the femoral head occurred concurrently 1 week after the initiation of steroids. As in the case of alcohol-induced osteonecrosis, adipocytes contained triglyceride vesicles. In rabbits treated with steroids, it was found that interosseous pressure was increased and the size of bone marrow fat cells was larger than in control rabbits.101 A histologic study of acetabular and proximal femoral bone in osteonecrosis of the femoral head revealed that osteonecrosis is more extensive in corticosteroid-induced compared with alcohol-induced or idiopathic osteonecrosis.102 The reason for this is unknown.
Table 103-5 Lipid-Altering Effects of Steroids and Alcohol
Coagulation and Osteonecrosis
The hyperlipidemia, increased serum free fatty acids, and increased prostaglandins that are associated with alcohol-induced osteonecrosis may potentially trigger vascular inflammation and coagulation. Other triggers for intravascular coagulation include atherosclerosis and arteriolar fibroid degeneration. Jones proposed that the progression of osteonecrosis from stage 1A to 1B is linked to an inability to clear procoagulants from blood or tissue.103 He proposed that decreased clearance of procoagulants leads to persistent levels of tissue thromboplastin, leading to arteriolar thrombosis, vascular stasis, free fatty acid–induced endothelial damage, and hypercoagulability. Studies have shown that patients with osteonecrosis had a much higher frequency of having at least one and at least two abnormal coagulant levels compared with normal controls. Of patients with osteonecrosis, 82% had at least one abnormal procoagulant level, and 47% had at least two. In normal controls, only 30% had one abnormal procoagulant level and only 2.5% had two or more. The procoagulants measured included free protein S, protein C, lipoprotein A, homocysteine, plasminogen activator inhibitor, stimulated tissue plasminogen activator, anticardiolipin antibodies (IgM and IgG), and resistance to activated protein C.104
In addition, both thrombophilia and hypofibrinolysis have been associated with osteonecrosis. Hypofibrinolysis leads to an increased likelihood of clot formation, and thrombophilia results in a decreased ability to lyse clots. This is yet another mechanism by which corticosteroids lead to osteonecrosis—high-dose steroids lead to increased plasma plasminogen activator inhibitor, decreased tissue plasminogen activator activity, and inhibition of the fibrinolytic pathway, thus leading to a higher risk for clot formation. There is an early indication that coagulation abnormalities may play a significant role in corticosteroid-induced osteonecrosis in SARS patients.105,106
Oxidative Stress and Osteonecrosis
Alcohol consumption is associated with reduced superoxide dismutase activity. Alcohol has deleterious effects on muscle including increased oxygen free radical–related damage, reduced myocardial contractility, defective mitochondrial function, and increased tissue enzymes.107 When rabbits were injected with methylprednisolone, elevations in 8-hydroxy-2′deoxyguanosine, a marker of DNA oxidative injury, were observed.108–110 This coincided with the development of osteonecrosis. A polymorphism in nitric oxide synthase, described later, was also associated with the development of osteonecrosis. This relationship between osteonecrosis and oxidative injury leads one to wonder if corticosteroid-induced osteonecrosis can be prevented or lessened in severity by simultaneous or prophylactic administration of antioxidants.
Nitric Oxide Synthase and Osteonecrosis
Glucocorticoids can cause derangements in vasacular responsiveness to vasoactive substances such as nitric oxide. Endothelial nitric oxide synthase (eNOS) stimulates the production of nitric oxide. Nitric oxide regulates vascular “tension” by acting as a vasodilator, inhibiting mononuclear adhesion to endothelial cells and preventing platelet aggregation. A defect in this activity can lead to increased vascular resistance and disruption to downstream blood flow, resulting in osteonecrosis.111
Multihit Hypothesis
Other proposed mechanisms involve endothelial cell injury,112 abnormal angiogenesis and repair mechanisms,113 the effects of vasoactive substances,114 activity of hepatic cytochrome P450 3A4,115 and intramedullary hemorrhage.116 Multiple mechanisms may be simultaneously occurring. Kenzora was the first to introduce the concept of cumulative stress.117 Corticosteroid-induced osteonecrosis seems to occur with greater frequency in patients who have significant underlying illness such as systemic lupus erythematosus118 or transplantation and less frequently or never in patients who are not chronically ill but are on steroids for an acute event such as head injury. Recent observations that corticosteroids induce osteonecrosis in SARS patients further support the notion that more than one insult to the bone or surrounding tissue may be necessary to precipitate osteonecrosis. For each of the known associations of osteonecrosis, different mechanisms may predominate such as lipid anomalies and apoptosis of osteoblasts in steroid-induced osteonecrosis, as well as elevated intraosseous pressures and coagulation abnormalities in dysbaric osteonecrosis, but additional factors may be necessary to precipitate osteonecrosis. The accumulated cell stress theory suggests that when the damaging effects of multiple events are added together, the involved bone is unable to recover from the chronic stress and osteonecrosis ensues.
Genetic Considerations
The degree to which genetics and the environment play in the pathogenesis of osteonecrosis is the subject of an ongoing investigation. Certainly, single nucleotide polymorphisms have been noted in a number of genes that may be associated with osteonecrosis. It has been argued that endothelial nitric oxide synthase is an important player in the development of osteonecrosis. Nitric oxide may have beneficial effects on three systems involved in osteonecrosis, namely skeletal, vascular, and thrombotic. Each of these may be targets for proposed mechanisms of pathogenesis of osteonecrosis. A comparative analysis of the 26-base pair repeat polymorphism in intron 4 and the Glu298Asp polymorphism in exon 7 of the eNOS gene in patients with idiopathic, steroid-induced, alcohol-induced, and normal control subjects was performed.119 The frequency of the homozygous 4a allele was found to be higher in patients with idiopathic osteonecrosis compared with control subjects. The frequency of the 4a/b allele was found to be higher in all types of osteonecrosis when compared with control subjects. The 4a allele is known to be associated with reduced synthesis of endothelial nitric oxide synthase, suggesting that nitric oxide may play a protective role against the development of osteonecrosis.
Forty-one percent of patients with osteonecrosis compared with only 20% of controls were homozygous for the 4G/4G mutation in the plasminogen activator inhibitor-1 gene.120 This mutation causes increased hypofibrinolytic plasminogen activator inhibitor activity, resulting in decreased stimulated plasminogen activator activity. This observation lends support to the theory that procoagulants may play a significant role in the pathogenesis of osteonecrosis. A polymorphism in the plasminogen activator inihibitor-1 (PAI-1) gene has also been reported to be predictive of osteonecrosis in children with acute lymphoblastic leukemia.121
Genetic variations in type and levels of lipoprotein (a) have been linked to osteonecrosis. Apo(a) is involved in lipid metabolism and the coagulation systems, and the Apo(a) low-molecular-weight phenotype is associated with an increased risk of osteonecrosis.122–124 Polymorphisms in the promoter for vascular endothelial growth factor (VEGF) and in the receptor for IL-23 were associated with osteonecrosis in the Korean population,125,126 reflecting the significance of the association of osteonecrosis with vascular disorders and autoimmune diseases, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree