Muscle Injuries
Traumatic muscle and myotendinous injuries were discussed in anatomic chapters. However, the complexity of these injuries and the fact that they may have features similar to other myopathies indicate that we should include them in this section, as well. Injuries may be due to overuse resulting in microtrauma, rhabdomyolysis due to intense exercise, focal acute tears, or delayed onset muscle soreness (DOMS).
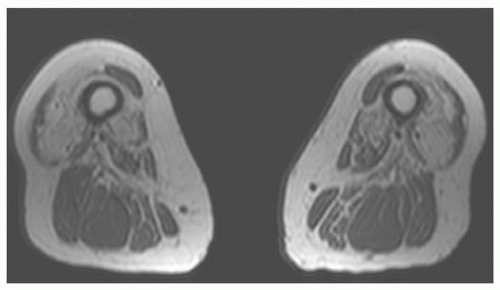
23,25,70,71,72,73,74,75,76Muscle injuries are graded depending upon the extent of injury. First-degree strains result in injury of a few fibers, second-degree injuries involve 50% of muscle fibers, and third-degree strains are complete disruptions of a muscle (
Fig. 15.12).
4,73,74,77 Minor injuries have excellent prognosis and usually are not imaged. DOMS is muscle pain related to exertion that occurs hours to days after the initiating activity.
70 This phenomenon is associated with eccentric (lengthening) contraction that results in ultrastructural damage, elevated plasma proteins, and edema on T2-weighted MR images. The extent of edema correlates with pain levels and elevations in creatine kinase.
70,74,77 Symptoms related to DOMS usually begin 1 to 2 days following exercise and usually improve over the next 3 to 4 days.
77 Symptoms are separated from grade 1 muscle strains clinically as in the latter the symptoms are acute and tend to resolve in 2 to 3 weeks.
73,77Muscle strains (grades 1 to 3) are classified due to the extent of injury. Trauma may be indirect or direct including laceration from penetrating injuries.
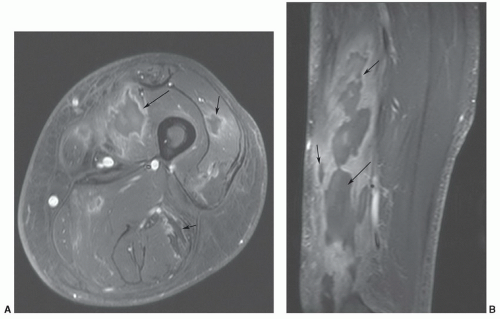
71,77 Grade 1 strains result in microscopic injuries with disruption of about 5%, but certainly less than 50% of muscle fibers. There is little loss of function and may be minimal swelling of the involved muscle on MRI. Edema and hemorrhage cause focal poorly defined high signal intensity on T2-weighted and STIR sequences. MR images in the axial and either coronal or sagittal plains define the extent of muscle involvement.
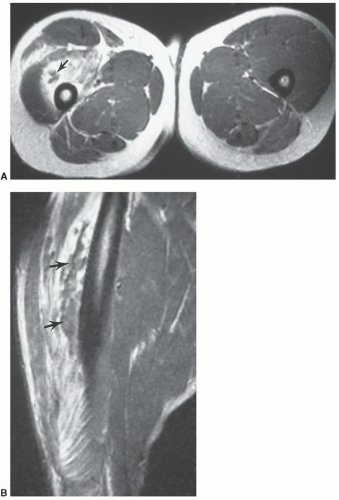
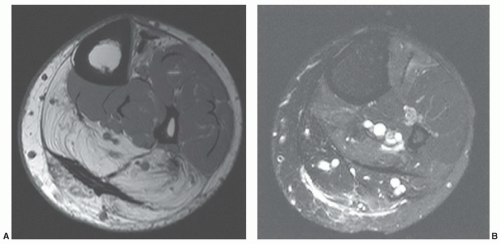
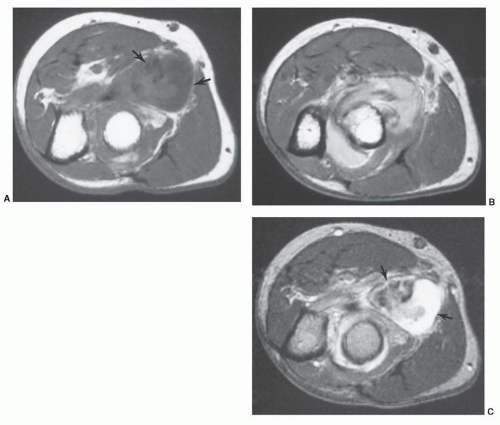
76,77Grade 2 strains result in loss of muscle function due to partial disruption (about 50% of the fibers) and frequently a hematoma at the myotendinous junction (
Figs. 15.12 and
15.13). Once again signal intensity is increased on water-sensitive sequences with variable signal intensity in an associated hematoma depending on the age of the lesion.
77 Contrast enhancement is useful when a hematoma is present to exclude underlying mass lesions. Hematomas enhance peripherally, whereas tumors tend to enhance centrally unless very necrotic.
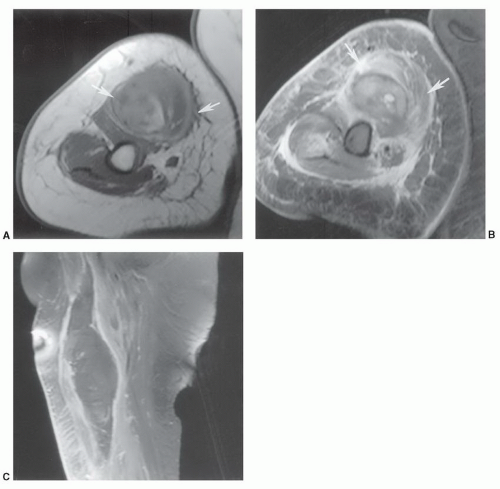
3,4,73,76,77Grade 3 strains result in complete disruption of the muscle or myotendinous junction. Depending upon the involved muscle, there may be significant retraction of the muscle fragments.
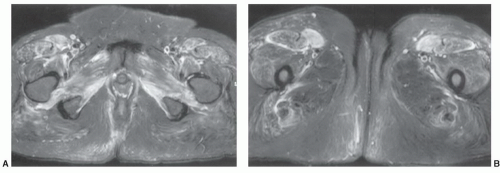
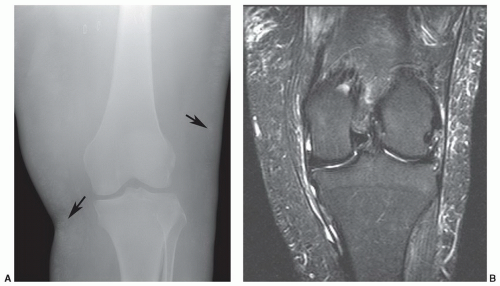
3,4,77 The site of injury almost always has a hematoma due to hemorrhage into the disruption site (
Fig. 15.14). Hematomas tend to heal more slowly and may require evacuation if adjacent to neural structures (
Figs. 15.14 and
15.15).
3Prognosis and repair vary with the extent of injury. Grade 1 strains similar to contusions typically heal within 2 weeks. Higher-grade strains may require 1 to 4 months to heal. Depending upon the activity (high-level athletes, etc.) operative intervention may be required for higher-grade strains and certain hematomas (
Fig. 15.14).
3,4,77Complications of muscle sprains include hernias, compartment syndrome, fibrosis, atrophy, and heterotopic ossification. Muscle hernias most frequently occur in the lower extremity. They are most common in athletes and soldiers. Hernias may be multiple and bilateral, with muscle protruding through the fascial defect. Muscle signal intensity is often normal on MRI. Although often palpable, it may be necessary to image muscle herniations with active muscle contraction. Therefore, fast scan techniques with motion studies or active muscle contraction may be required to confirm the diagnosis and exclude other soft tissue lesions. The axial image plane is often most useful for detection of hernias.
23,77Fibrosis is more common with grades 2 and 3 strains and more common in the calf than the thigh or upper extremities. Fibrotic tissue has low signal intensity on both T1- and T2-weighted sequences.
3,4,77 Larger hematomas may ossify or develop thick fibrous capsules with central fluid collections.
Compartment syndrome may occur acutely or be a chronic phenomenon due to increased venous flow and increased interstitial pressure in a give fascial unit.
77,78 Vascular and neural compression may occur with significant ischemic risk. Fasciotomy may be required if clinically measured pressures exceed 30 mm Hg.
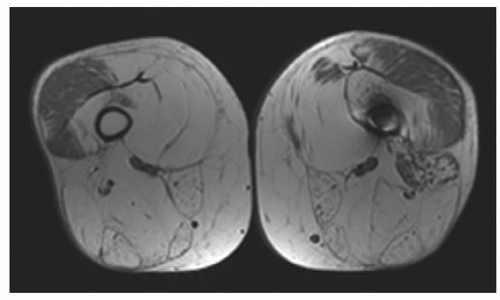
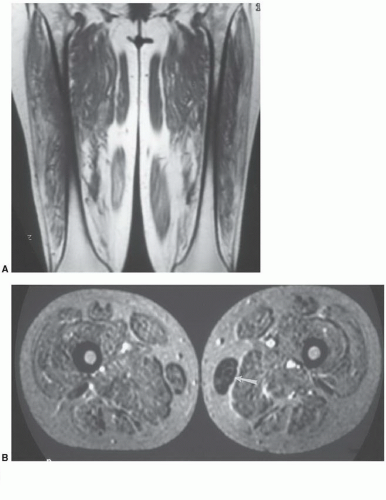
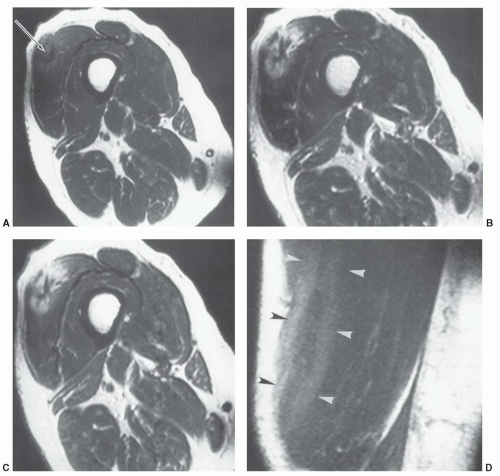
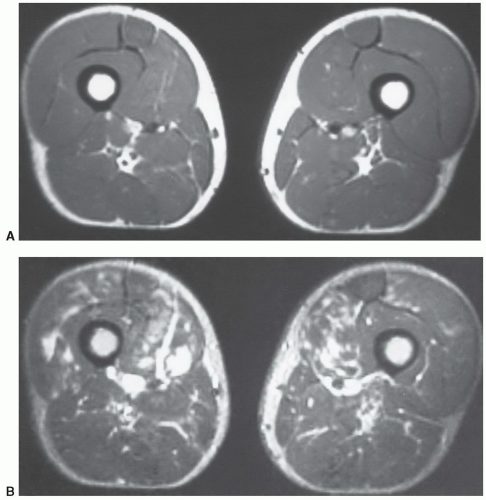
78Rhabdomyolysis may be due to ethanol overdose, infection, crush injuries, collagen disease, or intense exercise. MR images typically show a more generalized process than local muscle tears (
Fig. 15.16).
3,75,79Calcific myonecrosis is a rare sequelae of muscle trauma.
80 Knowledge of this condition is important, as clinical and radiographic features may mimic an aggressive neoplasm.
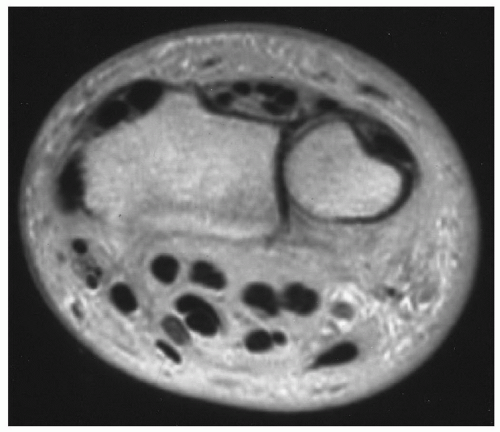
81,82 Calcific myonecrosis occurs with cystic muscle degeneration. The process can occur 10 to 64 years after trauma and results in a painful, expansive, calcified soft tissue mass. A remote history of compartment syndrome is common.
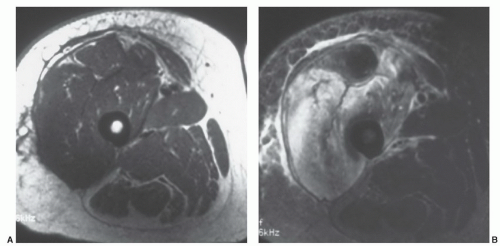
80,82Radiographs demonstrate plaque-like calcifications in the periphery of the mass. There may be adjacent bone erosion.
81 Increased tracer accumulation is evident on technetium-99m bone scans. The fluid-filled mass with
calcification is easily appreciated on CT studies. MRI demonstrates cystic changes on T1- and T2-weighted images. Calcifications may be more difficult to define if radiographs or CT are not available for comparison.
80,81 Contrast enhancement does not facilitate the diagnosis (
Fig. 15.17).
65Treatment typically requires debridement and excision of the entire region, with wound coverage using skin or muscle flaps. Secondary infection following treatment is common.
80,82
Morel-Lavallée Lesions
Morel-Levallée lesions result from abrupt separation of the skin and subcutaneous tissues from the underlying fascia.
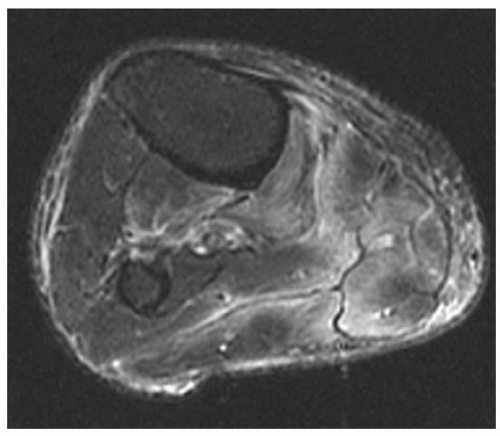
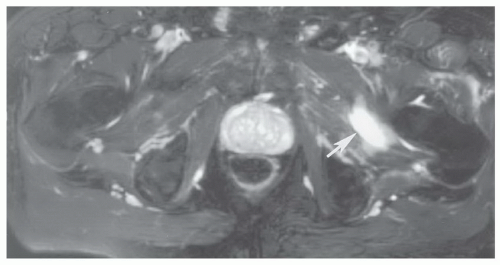
83,84,85 The lesions are most common near the greater trochanter and thigh (
Fig. 15.18), but have also been reported in the low back and knee.
85 Lesions may occur with a variety of traumatic etiologies though most are reported in football players and wrestlers.
85Injuries usually occur where the vascular plexus passes through the fascia. With disruption of the fascia, blood, lymphatic fluid, and tissue debris form the fluid collection. MR features are that of a fluid collection with low signal intensity on T1-weighted images and scattered areas of high signal intensity due to blood products. On T2-weighted images, the lesion is largely high intensity with mixed areas of intermediate to low signal intensity, based on the type of fluid and chronicity of the lesion (
Fig. 15.18).
83,84,85 The lesions are typically well defined and located between the fascia and subcutaneous fat.
85Some lesions respond to conservative therapy whereas others require aspirations to achieve resolution. Lesions that do not respond to these measures can be treated with doxycycline sclerodesis.
84