MR Unsafe
MR unsafe devices are devices known to pose hazards in any MR environment. This may be any ferromagnetic device or item.
Although evaluations are still in progress, many reports have defined a number of implants that may be potentially dangerous to the patient and/or affect image quality.
1,42,49,51,53,56,57,58,59,63 Cardiac pacemakers have always been considered a contraindication for MRI. Concerns regarding pacemakers include inhibition, asynchronous pacing, patient discomfort, heating, and motion sensation at the implant site.
73,74 In addition, significant image degradation may occur if the power pack is in the region being examined. Although some recent studies have demonstrated that pacemakers, implantable cardioverter defibrillators (ICD) show the potential that these devices may not be a
contraindication for MR, we and most other institutions continue to consider these devices as “MR unsafe.”
74In previous years, numerous heart valves have been studied at field strengths of 0.35 to 1.5 T. The artifacts created by most values were negligible, and it was concluded that patients with these prosthetic values can be safely imaged.
60,64 However, movement or torque was demonstrated with certain valves. In spite of this response to the static magnetic field, the deflection is minimal and not considered a contraindication.
56 More recently, prosthetic heart valves and annuloplasty rings have been studied at up to 3 T. The magnetic interactions are minimal even in elderly patients with age-related cardiac valve disease. Edwards et al.
75 postulated that the stiffened tissue in elderly patients makes it even more difficult to cause valve sutures to dislodge.
75,76 Therefore, hear valves and annuloplasty rings are not a contraindication for MR examinations. Patients with heart valves typically also have sternotomy
wires. Patients with sternotomy wires and retained epicardial pacemaker wires can be safely examined with MRI.
64Early studies of cerebral aneurysm clips demonstrated significant torque and risk to the patient when exposed to an MR environment.
47,56,66,67 Brown et al.
47 demonstrated that the strongest torque and most significant image artifacts were present with clips made of 17-7HP stainless steel. Titanium and tantalum clips showed the least attractive force and minimal image distortion. Subsequent studies at up to 8 T have provided more information regarding patient safety and aneurysm clips.
77,78,79 These studies also demonstrate that 17-7HP or 405 stainless steel aneurysm clips are an absolute contraindication for MRI.
77,78 Aneurysm clips made from titanium alloy, pure titanium, austentitic stainless steel, or materials considered nonferromagnetic or weakly ferromagnetic are not a contraindication for MRI.
77,78Most surgical clips at our institution are nonferromagnetic or contain minimal ferromagnetic material. Nonferromagnetic surgical clips pose no risk to the patient and MR examinations can be performed in the immediate post-operative period.
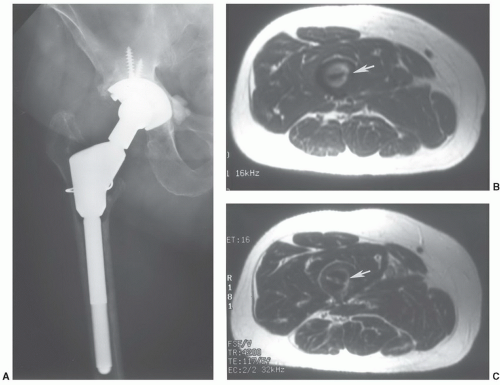
42 Nonferromagnetic or minimally ferromagnetic hemostasis clips may cause local image distortion, but do not pose a risk to the patient (
Fig. 3.2). Shellock and Spinazzi
42 recently reported several endoscopic hemostasis clips that may cause potential problems for patients undergoing MR examinations (
Table 3.2).
Other nonorth opedic metallic devices, including dental materials, ear implants, vascular filters and coils, genitourinary (GU) prostheses, ocular implants, and intrauterine devices, have also been investigated.
48,52,60,61 Some removable dental plates are ferromagnetic and cause significant local artifact (
Table 3.3).
48,57 These materials should be removed prior to MRI.
48 Permanent appliances such as braces may contain ferromagnetic material, but patients can be studied safely.
57 The facial region is not included on most musculoskeletal MR images. Therefore, artifacts from dental appliances usually are not a problem. We do not examine patients with permanent dental prostheses held in with magnetic posts as it is not yet clear what effect the magnetic field will have on these components.
1Cochlear implants are, for the most part a contraindication for MR examinations as the device and the patient may be at risk. Specific implants may be MR compatible at lower field strengths, but to date we consider all such devices as “MR unsafe.”
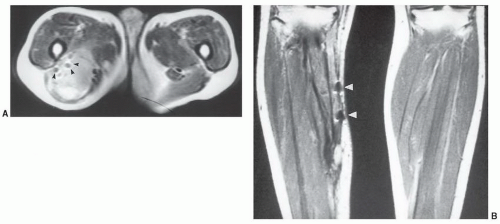
42,80GU prostheses and vascular filters and coils, because of their location, are more likely to create artifacts and need to be considered carefully (
Fig. 3.3). Most penile implants and GU sphincter prostheses do not contain significant ferromagnetic material. An exception is the Omni Phase (Dacomed) that contains significant amounts of metal.
79 Shellock
56 and Teitelbaum et al.
63 studied vascular coils, stents, and filters
in vitro and
in vivo (
Table 3.4). Their data indicate that artifact and torque are most significant with vascular appliances constructed with 304 and 316 stainless steel. Although the latter is not ferromagnetic in its original form, it does appear to undergo some change during configuration of appliances (i.e., Palmaz endostent). Materials creating minimal artifact include the appliances made with Beta-3 titanium, Elgiloy, nitinol, and mP32-N alloys.
63 Despite their magnetic susceptibility and the degree of artifact created, patients with these devices generally can be imaged safely. The ferromagnetic devices (Greenfield filter, constructed of 316L stainless steel) did not displace or perforate the inferior vena cava (IVC).
70 The position of devices containing ferromagnetic material [Gianturco embolization coil, Gianturco bird nest filter, Gianturco zigzag stent, Greenfield filter (316L stainless steel) and retrievable IVC filter, and Palmaz end ovascular stent] should be confirmed radiographically prior to examination. If the device is perpendicular to the magnetic field or immediately adjacent to the area of interest, the examination maybe inadequate and patient risk (due to change in position) is more significant.
63,71 Shellock and Shellock
69 evaluated 10 endovascular stents. They concluded that there was no significant interaction (motion or heating) with stents made of Elgiloy (cobalt, chromium, nickel, iron, and molybdenum), platinum-nickel, or tantalum.
Multiple new cardiovascular devices were also evaluated by Levine et al.
81 Permanent cardiac pacemakers, ICD, and heart valves were discussed previously. Therefore, we will focus on the other cardiovascular implants studied by these authors. Most coronary artery and peripheral vascular stents are configured of 316L stainless steel or nitinol. Other alloys
may also be used. However, most are nonferromagnetic or weakly ferromagnetic. Stents are implanted against the vessel wall and are well anchored. Although some studies suggest that MRI may be safer 6 to 8 weeks after the procedure, there is no strong clinical evidence to suggest delaying examination for this period is warranted. Therefore, most coronary and peripheral vascular stents come labeled as “MR safe” or “MRconditional.”
81 Large aortic stents are also nonferromagnetic or weakly ferromagnetic and due not pose a risk to the patient. However, the artifact generated by certain grafts [Zenith AAA endovascular graft (Cook), Endologix AAA stent (Endologix), and Lifepath AAA stent (Edwards Life-sciences Corp.)] cause significant susceptibility artifact rendering the aortic examinations inadequate.
81 IVC filters are configured of similar materials and may cause susceptibility artifact (
Fig. 3.3) but have not been demonstrated to pose a patient risk. Full incorporation to the vessel wall occurs in 4 to 6 weeks.
81 Care should be taken to include the product labeling in the patient record to avoid confusion regarding MR compatibility.
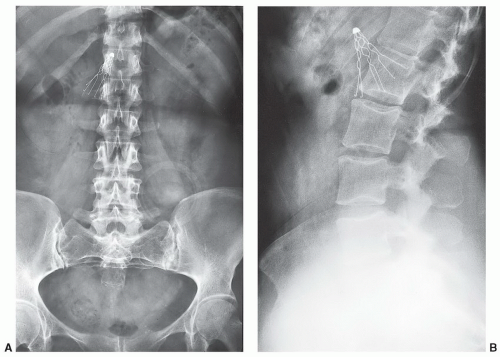
A potentially more difficult problem exists in patients with nonmedical foreign bodies or ferromagnetic material. Patients with shrapnel or other metal foreign bodies may not be aware that this material is present (
Fig. 3.4). In this setting, there is little that can be done until an artifact is detected during the examination. If the patient expresses concern over a potential foreign body, one should consider a radiograph or CT (orbital foreign bodies) of the area or, if clinically indicated, cancel the examination.
38 If an object is detected in the extremity and it is not near a nerve or in a region where its motion could cause damage, the examination can be performed with close monitoring
(technologist in the room, questioning the patient during and between pulse sequences). If the object has been present for 6 months or more, it will likely be fixated by scar tissue that reduces the risk of motion. Pigments in facial makeup (eyeliner, mascara, eye shadow) and certain tattoos may also contain ferromagnetic material (brown: ferric sulfate; flesh color: iron oxide; blue: cobalt chloride) and cause local heating and cutaneous inflammation.
55 Patients with tattooed eyeliner are particularly susceptible. These factors should be considered, and avoiding these examinations may be indicated.
Manufacturers of orthopedic appliances (plates, screws, joint prostheses, etc.) generally use high-grade stainless steel, cobalt-chromium, titanium, or multiphase alloys.
1 These materials are usually not ferromagnetic but may contain minimal ferromagnetic impurities. Orthopedic appliances at our institution have been tested for magnetic properties (torque or twisting in the magnet) and heating. No heating or magnetic response could be detected.
1,83 Davis et al.
49 also studied the effects of RF pulses and changing magnetic fields on metal clips and prostheses. No heating could be detected with small amounts of metal. Heating was demonstrated with two adjacent hip prostheses in a saline medium. However, it was concluded that metal heating in patients should not be a problem even with a large prostheses.
51,83 Heating and skin burns have occurred with looped or improperly positioned electrocardiogram (ECG) leads.
Nonferromagnetic metal materials may cause less significant artifacts on MR images and, if small enough, the insignificant area of signal void may be overlooked. The extent of metal susceptibility artifact created depends upon the size, shape, magnetic susceptibility, and field strength of the MR unit.
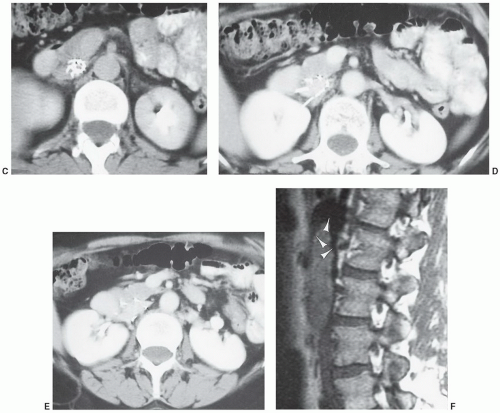
1,84 On low-field systems, some local distortion (
Fig. 3.5) may be seen. The extent of artifact is usually more obvious on high-field (≥1 T) systems (
Fig. 3.6). Many artifacts noted on MR images are due to local magnetic field distortions caused by both ferromagnetic and nonferromagnetic appliances. The degree of artifact is greater with the former. These distortions result in signal amplitude reduction near the metal surface.
69 The artifacts generally is oriented in the direct of the frequency readout gradient.
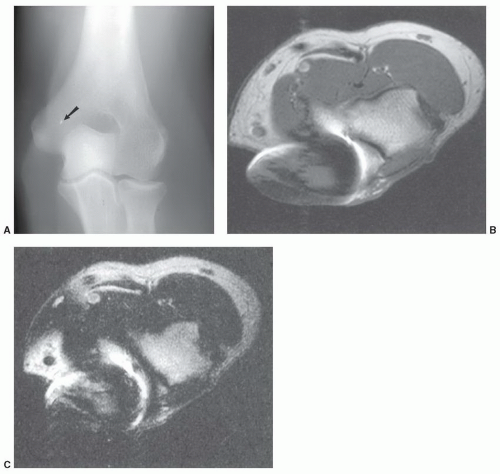
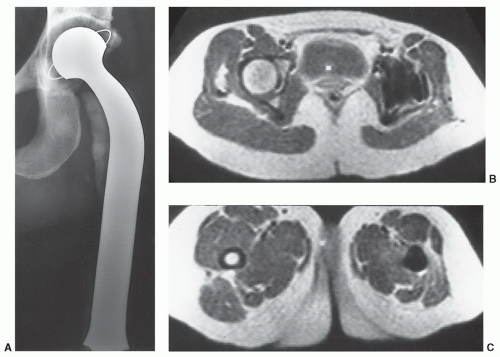
54,85,86The artifacts created by orthopedic devices vary significantly. When imaging a hip prosthesis, there are more artifacts created by the large, irregular head and neck than the more regular and smaller femoral stem (
Fig. 3.5). There are also more artifacts from metal screws and Harrington rods due to the contour irregularity of the implants
(
Fig. 3.7).
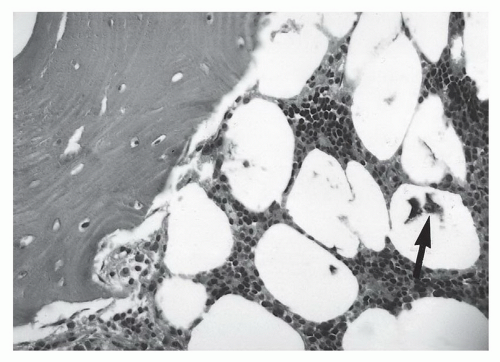
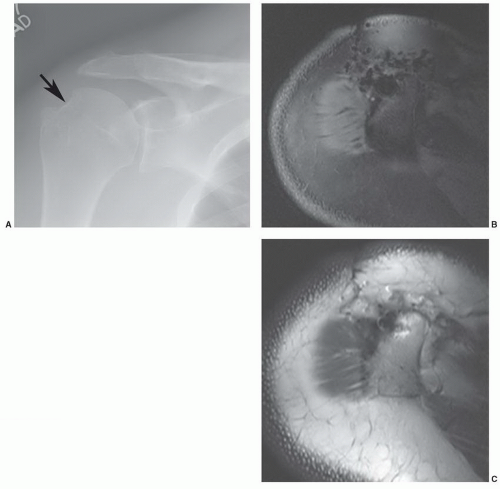
1,58 This may be due to the irregular contour (threads and ridges), screw position, or increased amounts of ferromagnetic impurities. Even small amounts of ferromagnetic materials can cause significant artifact.
87 For example, histological evidence has demonstrated metal debris in histiocytes around screw tracts and drill tracts, suggesting that small amounts of ferromagnetic material are present in screws used for internal fixation. Drill bit fragments may also contribute to screw tract artifacts. A fibrohistiocytic response normally occurs around these screws if they are left in place for a significant length of time (
>3 months). This phenomenon may allow artifacts to occur even though no significant metal can be detected on routine radiographs (
Fig. 3.7).
In recent years, multiple MRI parameter changes have been tested to reduce metal artifact.
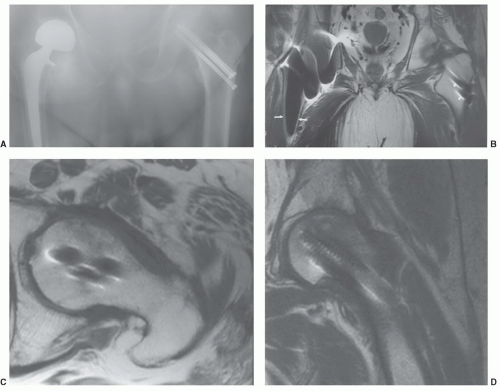
84 As 120,000 total hip procedures are performed each year in the United States, much of the effort has been directed at imaging of patients with hip arthroplasty (
Figs. 3.5 and
3.6).
8,85,86,87,88,89,90,91,92 Many of the same parameter modifications can be used regardless of the types of prostheses or fixation devices present.
87,88,89,90,91,92 Parameter modifications include changes in the use of conventional pulse sequences (some improve and some exaggerate the artifact), slice thickness, matrix, band width, view angle tilting (VAT), newer metal artifact reduction sequences (MARS).
87,88,89,90,91,92 Metal artifact is also increased with increased field strength.
90Certain conventional pulse sequences demonstrate reduced artifact whereas others increase metal artifact. Short TE-(echo time) or T1-weighted spin-echo (SE) sequences reduce artifact. Fast short TI inversion recovery (STIR) sequences serve to increase signal intensity near the metal implant.
87 Also, use of water excitation instead of fat suppression not only reduces artifact but also reduces imaging time by up to 50%.
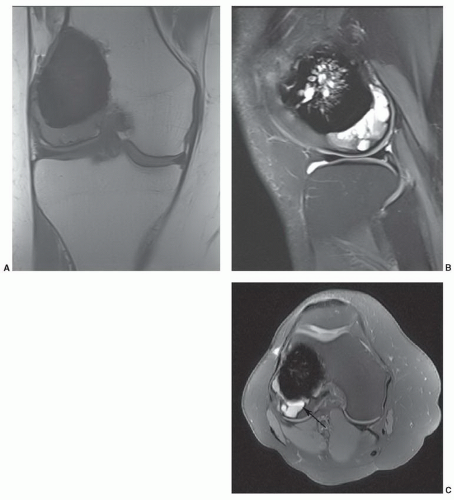
85Fast spin-echo (FSE) and gradient-echo (GRE) sequences create more artifacts about the implant. Blooming occurs about even minute metal debris with GRE sequences (
Fig. 3.8).
90,91,92 This misregistration artifact occurs only in the frequency-encoding direction. Misregistration artifact is proportional to the field inhomogeneity and inversely proportional to the frequency-encoding
gradient strength (
Fig. 3.8). Artifact reduction can be achieved by increasing the frequency-encoding gradient and altering the frequency-encoding direction so that it is parallel to the metal object.
90 This is often difficult to accomplish due to the multidirectional positions of plates and screws and rod and pedicle screw implants.
Increasing the bandwidth from 16 to 64 mHz has been shown to decrease the artifact about hip implants significantly (
Fig. 3.9).
87,90,91 Increasing the matrix size (equal to or greater than 256 × 256) and the number of acquisitions also decreases local image distortion around metal implants.
91Additional techniques for artifact reduction include VAT and MARS.
90,92 VAT technique applies a compensatory gradient during image acquisition that corrects for local field inhomogeneity. MARS combines increased bandwidth and an added gradient during acquisition. These parameters are applied concurrently with the frequency-encoding gradient (Gx) and in the same direction and amplitude as the slice gradient (Gz). This sequence results in some loss of SNR, but reduces image blurring significantly.
92Methyl methacrylate and bone graft material is used to fill medullary cavities as well as to cement components. With the former, artifacts are minimal on MR images. Methacrylate is seen as a dark area of no signal, but there is no image distortion (
Fig. 3.10).
External fixation devices may be bulky, but most are not ferromagnetic as the materials are similar to those used in internal fixators.
1 Magnetic properties can be easily checked with a hand-held magnet prior to the MR examination. The extent of image degradation will vary with the magnetic susceptibility. Recently, manufacturers of fixators have begun to use nonferromagnetic material.
1 Table 3.5 lists the materials used for external fixation devices in order of increasing artifact production on MR images.
49 In addition to the artifact potential, coil selection may also be restricted due to the size of these fixation systems. This can result in a decreased SNR and reduced image quality. However, most patients can be examined using the head or body coils.
Evaluation of patients with casts and bulky dressings (Robert-Jones, etc.) is also possible with MRI. Although coil selection is more restrictive, we have not detected any reduction in image quality because of these materials.
1