15 Fibroblasts and Fibroblast-like Synoviocytes
What is a Fibroblast?
Fibroblast Identity and Microenvironments
Tissue-resident macrophages in the liver (Kupffer cells) and lung (alveolar macrophages) perform very different functions compared with macrophages in the brain (glial cells) or skin (Langerhans cells), yet they are all members of the monocyte/macrophage family. Until recently fibroblasts had been thought of as ubiquitous, generic cells with a common phenotype even within different tissues. However, we now know that fibroblasts from different organs are more like their macrophage counterparts, with unique morphology and repertoires of ECM proteins, cytokines, co-stimulatory molecules, and chemokines specialized to the different microenvironments in which they are found. This also extends to their function as “immune sentinel” cells, expressing innate immune system pattern recognition receptors such as Toll-like receptors (TLRs), which trigger a proinflammatory response when ligated by bacterial or viral determinants. When fibroblast transcriptional profiles are examined using microarray techniques, fibroblasts hold a strong memory of their anatomic position and function in the body. Early studies demonstrated that fibroblast transcriptomes (the global picture of transcribed genes measured using microarrays) could be clustered into peripheral (synovial joint or skin fibroblasts) versus lymphoid (tonsil or lymph node) groups according to their organ of origin, with the potential to shift their transcriptional profiles by treatment with inflammatory mediators such as tumor necrosis factor (TNF), IL-4, or interferon-γ. More extensive analysis of expression profiles from primary human fibroblasts by Rinn and colleagues has shown large-scale differences related to three broad anatomic divisions: anterior-posterior, proximal-distal, and dermal-nondermal. Genes involved in pattern forming, cell-signaling, and matrix remodeling were found to predominantly account for these divisions.1 The gene expression profile of adult fibroblasts may therefore play a significant role in assigning positional identity within an organism. More recently, it has become clear that these stable changes in gene transcription are brought about through epigenetic activation and silencing of the HOX family of landscaping genes.2 Such epigenetic patterning, whereby covalent modifications are made to regulatory regions of DNA, or to the histones around which the DNA is wrapped in order to control access of transcriptional complexes, is a prototype for the stable changes that are also seen in fibroblasts. Epigenetic modifications result in stable changes in gene expression that persist over cellular generations in the absence of mutation of the primary DNA sequence, and which therefore drive the persistence of disease, as is described in more detail in Chapter 22.
Embryologic Origins
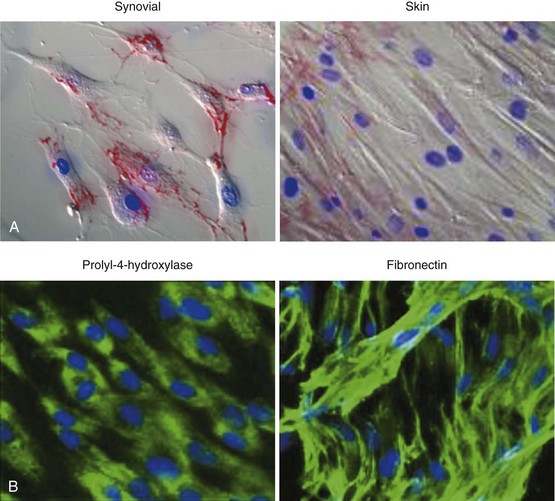
The problem of distinguishing fibroblasts of differing origin or maturity has historically been difficult due to a lack of specific cell surface markers. Whereas cluster of differentiation (CD) markers have revolutionized the isolation and study of leukocyte subsets, there have been relatively few, poor-quality discriminatory markers allowing the identification of fibroblast subpopulations. Fibroblasts have therefore traditionally been identified by their spindle-shaped morphology (Figure 15-1), elaboration of ECM, and lack of positive markers for endothelium, epithelial, and hemopoietic cells.
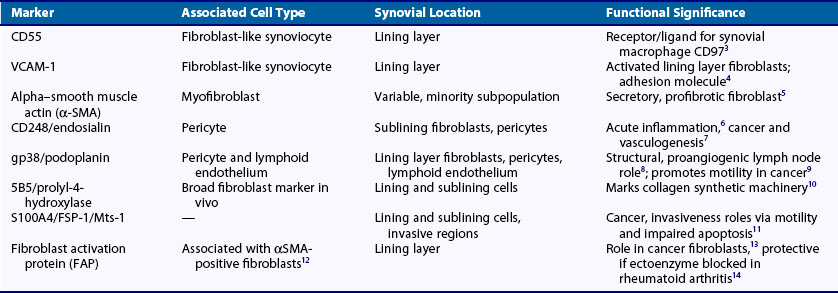
However, there is growing evidence that fibroblasts are not a homogeneous population but exist as subsets of cells, much like tissue macrophages and dendritic cells. It is likely that connective tissue contains a mixture of distinct fibroblast lineages with mature fibroblasts existing side by side with more immature fibroblasts that are capable of differentiating into other connective tissue cells. Recent studies have begun to identify novel markers that demarcate distinct subpopulations of stromal cells during development and which have the potential to act as markers for different subpopulations of fibroblasts, each with different roles. Such markers include smooth muscle actin, which marks out a population of secretory, activated cells termed myofibroblasts, and more recently discovered markers such as CD248 and gp38 (podoplanin) (Table 15-13–14 and see text later). Fibroblasts have been defined in terms of their embryologic origins and lineage relationships and are generally considered to be mesenchymal in origin. However, cell populations that appear to blur the distinction between hemopoietic and nonhemopoietic populations have now been identified. In addition, other unexpected shifts in lineage have been reported including differentiation from neural stem cells into myeloid and lymphoid hemopoietic lineage. Classification by such lineages is therefore becoming increasingly untenable.
Origins of Fibroblasts in Tissue
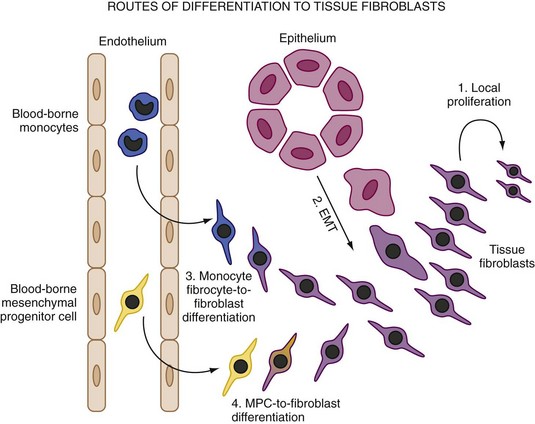
Both inflammation and wound healing are characterized by the formation of new tissue. However, recent findings suggest that the new cells that form the remodeled tissues are not necessarily, as was hitherto assumed, derived from the proliferation of cells that are resident in the adjacent noninjured tissue. This is an important issue because in both RA and fibrotic pathologic conditions, fibroblasts accumulate in excessive numbers despite apparently low proliferative rates. The principle origin for fibroblasts is from primary mesenchymal cells and that on appropriate stimulation fibroblasts can proliferate locally to generate new fibroblasts. In fact, though an increase in fibroblast numbers caused by local proliferation does occur, fibroblasts may arise from other sources (Figure 15-2). The first of these is local epithelial to mesenchymal transition (EMT). This is an essential, physiologically important developmental mechanism for diversifying cells in the formation of complex tissues. However, fibroblasts also appear to be derived by this process in adult tissue following epithelial stress such as inflammation or tissue injury. EMT both disaggregates epithelial cells and reshapes them for movement. The epithelium loses polarity as defined by the loss of adherens junctions, tight junctions, desmosomes, and cytokeratin intermediate filaments. Epithelial cells also rearrange their F-actin stress fibers and express filopodia and lamellipodia. A combination of cytokines and matrix metalloproteinases (MMPs) associated with digestion of the basement membrane is believed to be secreted and important in the process. The transition of epithelial to mesenchymal cell populations has been shown to occur in cancer and in diseases of the lung and kidney in which the process has been implicated in fibrotic disease.15 Early evidence suggests that a similar process may occur within the RA synovium.16
An alternative explanation for the accumulation of stromal cells in chronic inflammatory conditions such as rheumatoid arthritis (RA) lies in the possibility of blood-borne precursors. In the mid 1990s it was shown that vascular precursors (angioblasts) could be found circulating in the blood of normal individuals and that they could be recruited to sites of vasculogenesis in a rabbit ischemic hind limb model.17 This demonstrated that circulating mesenchymal precursors exist outside the hemopoietic system. Subsequent work has confirmed the presence of circulating cells of a mesenchymal phenotype in human subjects. These cells bear a remarkable resemblance to the synovial fibroblasts found in the joints of patients with RA, which accumulate in large quantities in the joint lining despite little evidence of proliferation. Interestingly, Marinova-Mutafchieva and colleagues18 showed that an influx of such cells preceded inflammation in a mouse collagen-induced arthritis model, suggesting that there may be a role for blood-borne stromal cell precursors in the initiation of inflammatory diseases. Furthermore, evidence now exists to show that synovial fibroblasts themselves may migrate in the bloodstream, at least between distant sections of human cartilage in SCID mice,19 raising an intriguing parallel to cancer and the radical concept of RA as a metastatic disease of the stroma.
Another circulating precursor cell that could account for the accumulation of fibroblasts in disease is the fibrocyte. Fibrocytes appear to comprise 0.1% to 0.5% of nonerythrocytic cells in peripheral blood and have been shown to rapidly enter sites of tissue injury and contribute to tissue remodeling in models of inflammatory lung disease.20 They are adherent cells with a spindle-shaped morphology that express MHC class II and type I collagen and which arise from within the CD14+ (monocyte) fraction of peripheral blood.21 Fibrocytes are capable of matrix elaboration and differentiate along fibroblast lineages under the influence of cytokines, particularly transforming growth factor-β (TGF-β). The mere fact that a cell type apparently arising from within the monocyte lineage may become a “mesenchymal” stromal cell such as a fibroblast implies a further degree of plasticity and blurring of the apparently clear dividing line previously thought to exist between hemopoietic and nonhemopoietic lineages.
Fibroblasts versus Mesenchymal Progenitor Cells
The potential role of circulating mesenchymal cell precursors (variously termed mesenchymal stem cells [MSCs], mesenchymal stromal cells, or mesenchymal progenitor cells [MPCs]) as sources of tissue fibroblasts is highlighted by the remarkable capacity of these cells to differentiate into other members of the connective tissue family including cartilage, bone, adipocyte, and smooth muscle cells. This ability was initially demonstrated in bone marrow stromal cells, RA synovial fibroblasts, and circulating mesenchymal cells. Therefore it was suggested to define a characteristic mesenchymal phenotype on the basis of the hypothesis that the rheumatoid synovium could become populated by a large proportion of circulating mesenchymal progenitor cells exported from the bone marrow. However, the property of trilineage differentiation (“pluripotentiality”) has now been shown to be a property of many adult tissue fibroblasts, though varying somewhat between fibroblasts from different tissues, implying a hitherto unsuspected degree of plasticity in the body’s stromal cell populations.22 The two previously separate fields of mesenchymal precursor cell biology and largely disease-centered fibroblast biology have therefore rapidly converged. However, the concept of bone marrow stromal precursors remains interesting; in chimeric murine models with bone marrow GFP expression, arthritic joints contained significantly more GFP-positive+ cells than nonarthritic joints, supporting a bone marrow origin for expanded fibroblast populations.23
Physiologic Characteristics and Functions of Fibroblasts
Production of ECM Components
Ensuring the homeostasis of the ECM is one of the primary functions of fibroblasts. In order to do this, fibroblasts must be capable of both producing and degrading ECM, as well as adhering to and interacting with existing matrix components. Fibroblasts produce a number of ECM molecules, both fibrous proteins and polysaccharide gel components such as collagens, fibronectins, vitronectin, and proteoglycans, which are then assembled into a three-dimensional network. This provides a framework through which other cell types, which use varying strategies to navigate through the ECM, can move24 and also provides a substrate for the deposition of haptotactic (tissue rather than fluid-based) gradients of chemokines and stores of growth factors to direct cellular movement and behavior in a regional fashion.25 The types of ECM molecules produced by individual populations of fibroblasts differ from tissue to tissue, reflecting the diversity of fibroblasts in different organs. For example, dermal fibroblasts produce significant amounts of type VII collagen, which adheres the epidermal and dermal layers in the skin. Fibroblasts in other organs such as the lung and kidney produce mainly interstitial, fibrillar collagens (particularly types I and III).
Attachment to and Interaction with Extracellular Matrix
Integrins
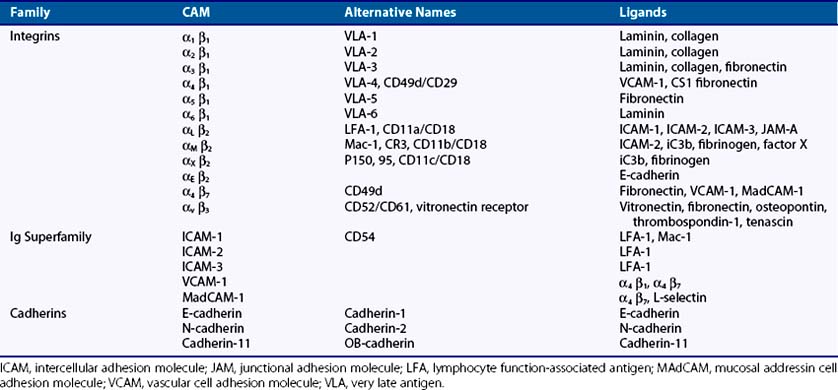
Integrins are key mediators of both cell-to-matrix and cell-to-cell adhesive interactions. They are expressed as transmembrane heterodimers containing one α- and one β-subunit, of which at least 25 αβ combinations are known (Table 15-2). α1β1 and α2β Integrins are the main adhesion molecules responsible for the attachment of fibroblasts to collagen, while other β1 integrins such as α4β1 and α5β1 integrins mediate attachment of fibroblasts to fibronectin and its spliced variants. In addition, αv integrins are responsible for attachment to vitronectin.
Syndecans
In addition to conventional integrin-to-ligand binding, additional accessory molecules allow for the integration of adhesive contacts and local growth factor signaling. Syndecans are a family of four single transmembrane domain proteins that carry three to five heparan sulfate and chondroitin sulfate chains, allowing for interaction with a large variety of ligands including fibroblast growth factors, VEGF, TGF-β, and ECM molecules such as fibronectin.26 Syndecans are expressed on fibroblasts in a tissue-specific and development-dependent manner. Data from syndecan knockout mice indicate that syndecan-4 is involved in wound healing, and the response of syndecan-4- deficient fibroblasts to fibronectin attachment is significantly altered.27
Immunoglobulin Superfamily Receptors
The immunoglobulin superfamily is a diverse group of transmembrane glycoproteins defined by the presence of one or more immunoglobulin-like repeats of 60 to 100 amino acids with a single disulfide bond.28 While including numerous adaptive immune system genes (immunoglobulins, T cell receptor, major histocompatibility complex), adhesion proteins such as ICAMs 1 to 3, VCAM-1, and MadCAM mediate both cell-to-cell interactions and adhesive interactions with integrins (see Table 15-2).
Cadherins
Cadherins mediate homotypic, calcium-dependent adhesive interactions with the same cadherin species expressed by neighboring cells.29 Classical cadherins possess five extracellular domains, a single-pass transmembrane domain, and a highly conserved cytoplasmic tail. The cytoplasmic tail interacts with β-catenin, which in turn binds α-catenin, forming a linkage between the cadherin-catenin complex and the actin cytoskeleton. Tightly regulated expression of cadherins is essential to embryogenesis but is also critical for tissue morphogenesis and tissue-specific cell differentiation. Cadherins also modulate cell proliferation and invasion through activation of intracellular signal transduction pathways, modulation of matrix metalloproteinase production, and association with growth factor receptors.30–32
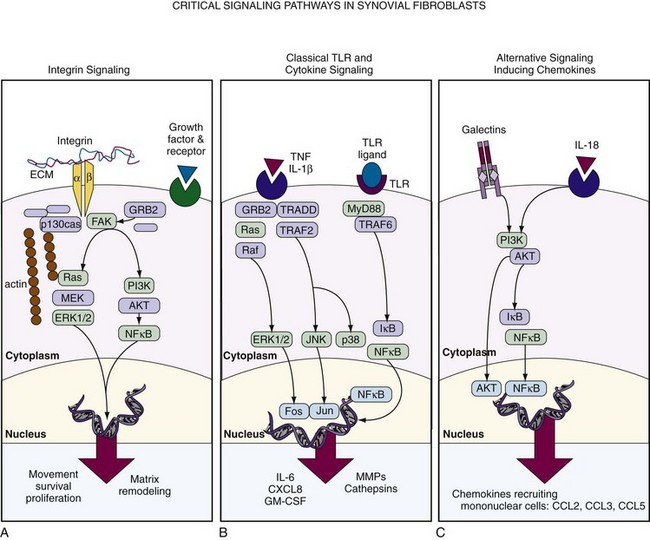
Importantly, interaction with adhesion molecules not only regulates adhesion and motility but also directly influences activation status, apoptosis, and proinflammatory and anti-inflammatory responses in fibroblasts and other cells. The engagement of cell adhesion molecules such as integrin receptors on the surface of fibroblasts results in the formation of focal adhesion complexes, which activate intracellular signaling cascades regulating cell proliferation and survival, the secretion of certain cytokines and chemokines, and matrix deposition and resorption. In particular, integrin-to-fibronectin engagement induces matrix metalloproteinase expression, linking adhesion-to-matrix remodeling33 (Figure 15-3). Among the signaling molecules that transmit signals from the integrins to the cell interior, focal adhesion kinase (FAK) plays a central role.34 FAK, a tyrosine kinase, is recruited into newly established focal contacts and, in turn, recruits other adapter proteins such as p130Cas and Grb2. This leads to the activation of phosphatidylinositide 3-kinase (PI3K) and Src-kinase and promotes the initiation of a variety of signaling cascades, culminating in the ERK MAPKinases and activation of transcription factors. Such pathways can also be activated through FAK-independent signaling events, such as through growth factor receptor ligation. The exact mechanisms by which different signals cooperate to mediate a specific response of fibroblasts and how this translates into distinct pathologies are not yet fully defined.
Degradation of Extracellular Matrix by Fibroblasts
Remodeling of the ECM requires fibroblasts to express an extensive repertoire of matrix-degrading enzymes with varying specificity. Although these are crucial to tissue maintenance and repair, inappropriate overexpression of such enzymes is a key factor in the joint damage, particularly to cartilage, which occurs in inflammatory disease. Such enzymes fall into a number of families including matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), cathepsins, and aggrecanases. These are covered in detail in Chapter 8.
With the exception of MMP-2 and the MT-MMPs, which are constitutively expressed by fibroblasts, MMP expression is regulated by extracellular signals via transcriptional activation in fibroblasts. Three major groups of inducers can be differentiated: proinflammatory cytokines, growth factors, and matrix molecules. Among the cytokines, IL-1 is perhaps the most potent inducer of a variety of MMPs including MMP-1, MMP-3, MMP-8, MMP-13, and MMP-14. Fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) are also known inducers of MMPs in fibroblasts because they potentiate the effect of IL-1 on MMP expression. All MMP promoter regions except MMP-2 contain activator protein-1 (AP-1) binding sites; however, there is good evidence that all MAPKinase families (ERK, JNK, and p38 pathways) (see Figure 15-3), in addition to activators of NFκB, STAT, and ETS transcription factors participate in MMP regulation.35–39 Matrix proteins (collagen, fibronectin) and especially their degradation products also activate MMP expression in fibroblasts, providing the possibility for site-specific MMP activation in regions of matrix breakdown.40
Fibroblasts as Innate Immune Sentinels
Classically, macrophages have been studied as sources of inflammatory cytokines and chemokines in response to innate immune stimuli and portrayed as immune sentinel cells accordingly. However, when activated by substances released during tissue injury or the products of invading microorganisms, fibroblasts are capable of elaborating a broad repertoire of inflammatory mediators, which fully justifies their classification as immune sentinel cells. Through expression of TLRs 2, 3, and 4, fibroblasts respond to bacterial products such as LPS by activating the classical NFκB and AP-1 inflammatory pathways, generating chemokines capable of recruiting inflammatory cells, and generating metalloproteinases capable of degrading matrix.41–43 However, TLR expression may be increased by proinflammatory cytokines TNF and IL-1β within the local microenvironment44 and may also be activated by endogenous cellular debris such as necrotic cells in synovial fluid, leading to widespread fibroblast activation in disease.45 As immune sentinels, fibroblasts are capable of bridging the innate and adaptive immune responses through expression of the molecule CD40. This molecule was initially assumed to be restricted in its expression to antigen-presenting cells such as macrophages and dendritic cells. However, it is widely expressed by fibroblasts within discrete tissues. Engagement of CD40 by its ligand CD40L expressed on a restricted population of immune cells including activated T lymphocytes is critical for the further induction of proinflammatory cytokines and chemokines during an immune response, as well as for antibody production by CD40-expressing B lymphocytes. Fibroblasts also need to be able to respond to more generic danger signals. The intracellular apparatus for response to danger signals such as high levels of urate has recently been identified as the NOD-like receptor family made up of NOD (nucleotide-binding oligomerization domain) and NALP (NACHT domain, leucine-rich-repeat [LRR] domain, and pyrin domain [PYD]-containing protein) receptors. A high local level of urate released by dying cells triggers formation of the active NALP3 inflammasome complex, which results in release of IL-1.46 Expression of high levels of NOD-2 and NALP3 (cryopyrin) are seen in the RA synovium and can be induced in fibroblasts by TLR ligands and/or TNF,47,48 although their relationship with as yet unidentified ligand molecules remains unclear; the NOD-2 ligand muramyl dipeptide is, however, present in the bowel, where it has a role in driving inflammation in Crohn’s disease.
Role of Specialized Fibroblast Subsets within Tissue Microenvironments
Combining surface markers with consistent function has been the key to decades of development in the field of leukocyte biology. By comparison, stromal cell biologists have had remarkably few such stable markers. However, this situation is now gradually changing and certain areas of developmental biology have spearheaded identification of putative markers (such as CD248) through approaches such as immunization of animals with human fibroblasts and digesting and identifying stromal cell subpopulations in tractable organ systems. One such example is the murine thymic stroma, in which subsets with both geographic and functional consistency have been identified. For instance, Link and colleagues49 identified CD45−, gp38-positive cells, which were not lymphatic endothelium (CD31−) in the thymus as T-zone fibroblastic reticular cells. This population of cells geographically restricted to the T zone provides a limited pool of essential homeostatic survival factors, IL-7 and CCL19, for T lymphocytes, serving a key niche function for which adaptive immune cells must compete.49
A further subpopulation of specialized fibroblast-like cells of mesenchymal origin is the pericyte. These cells ensheath small blood vessels (arterioles, capillaries, and venules) and are involved in vasculogenesis, matrix stabilization, and immunologic defense. Pericytes have been hypothesized to represent the extralymphoid source of mesenchymal progenitor cells and express markers consistent with mesenchymal stem cells. Their further definition with newer stromal cell markers will be able to establish a mesenchymal progenitor cell niche.50
Fibroblast-like Synoviocytes in the Normal Synovium
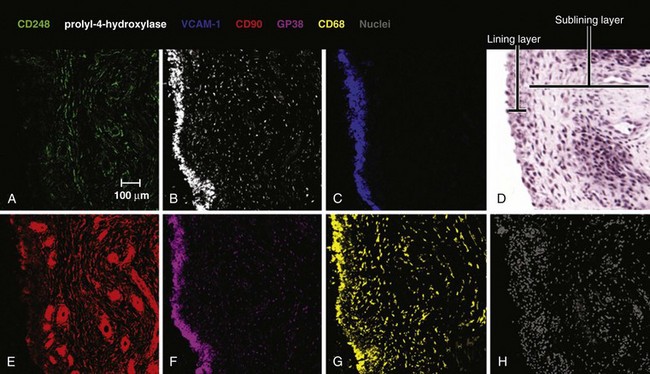
The normal synovium provides an excellent prototypic model of fibroblast subsets defined by known markers, some of which are responsive to disease. In health the synovium is a delicate, thin structure attaching bone and the joint capsule. It is divided into two layers. One is a two- to three-cell thick lining layer, which is formed in roughly equal proportions of CD68+, phagocytic type A macrophage like synoviocytes and type B mesenchymal, fibroblast-like synoviocytes (FLS). This layer must subserve a barrier function, and FLS must secrete lubricative substances including hyaluronic acid and lubricin, as well as secreting the lining layer matrix. The second layer is the sublining layer, which is composed of less densely packed fibroblasts and macrophages in a loose tissue matrix along with blood vessel networks. FLS in the lining layer are associated with a number of cellular markers (see Table 15-1) including CD55 (decay accelerating factor [DAF]), VCAM-1 (which outside T cell–to-integrin interactions is generally only expressed by bone marrow fibroblasts providing support for the B cell developmental niche51), uridine diphosphoglucose dehydrogenase (UDPGD) reflecting the ability to synthesize hyaluronan, and the novel marker gp38.52 Sublining FLS are instead marked by the nonspecific cellular marker CD90 (Thy-1), which also recognizes endothelium, and by the recently discovered marker CD248, which marks both pericytes and stromal fibroblasts. Gp38 marks cells in the sublining region including lymphatic endothelium and pericytes (Figure 15-4). As mentioned earlier, the unique lining layer barrier function is supported not by a basement membrane and conventional tight junctions but by homotypic interactions between cadherin-11 molecules.53 Randomly assorted cells expressing classical cadherins such as cadherin-11 will sort themselves in a cadherin-specific manner, emphasizing their importance in the generation and maintenance of organ integrity. Cadherin-11 mediates selective association of mesenchymal rather than epithelial tissues, a function that is carried forward after embryogenesis in structures such as the joint lung and testis.54 Cadherin-11 knockout mice exhibit a hypoplastic synovial lining that lacks the normal numbers of synovial lining cells and is deficient in ECM quantity.55 Adhesion between type A and type B synoviocytes is maintained by ICAM-1:β2 integrin and VCAM-1:α4β1 integrin interactions.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree