Layer
Anatomy
Pathoanatomy
I Osteochondral
Femoral head
Cam impingement
Acetabulum
Pincer impingement
Sub-spine (AIIS) impingement
II Inert
Joint capsule
Instability
Labrum
Labral tear, degeneration, ossification
Ligamentum teres
Ligamentum teres tear
III Contractile
Musculature crossing hip
Muscle strain
Musculature crossing lumbosacral spine
Muscle tear
Musculature crossing pelvic floor
Tendinopathy
IV Neuromechanical
Neurovascular structures
Nerve injury
Axial/appendicular coordination and mechanics
Spine and lower extremity malalignment
Pain syndromes
Table 2
Potential nerves injured and mechanisms during hip arthroscopy
Nerve | Mechanism |
|---|---|
Pudendal | Pressure due to perineal post |
Lateral femoral cutaneous | Direct injury due to portal placement |
Common peroneal | Traction |
Sciatic | Traction or portal placement |
Femoral | Traction or portal placement |
Superior gluteal | Portal placement |
Complications
Iatrogenic Chondrolabral Injury
The overall incidence of iatrogenic chondral and labral injury during hip arthroscopy is 3.8 % and 0.9 %, respectively, but have been reported to be as high as 20 % and 20 %, respectively [7, 13]. To obtain joint access, sharp instrumented joint entry is required for visualization, instrumentation, and mobilization. Unintentional injury to the labrum or articular cartilage may occur during initial portal placement from spinal needle entry, dilation, cannulation, or capsulotomy. During the early learning curve of hip arthroscopy, the rate of iatrogenic chondrolabral injury is greater with earlier time points [5]. Although there is published literature demonstrating that iatrogenic labral punctures have no significant effect on short-term clinical outcome [13], various studies have shown improved results with labral preservation compared to excision/debridement, and longer-term studies might shed further light on this subject [14–16]. Other recent investigations offer techniques to achieve a very low rate of chondrolabral injury (Table 3) [17, 18]. The latter recommend positioning the hip in mild flexion (15–20°), internal rotation, adduction, and traction to break the suction seal and achieve approximately 10 mm of distraction (Fig. 1). The safety of a blind anterolateral portal usually makes it the initial portal placed. A 70° arthroscope is used to directly visualize extra-labral anterior portal placement. The arthroscope is switched to the anterior portal to verify that the anterolateral portal is extra-labral. The arthroscope is switched back to the anterolateral portal, and a transverse interportal capsulotomy is made. This step requires precision to avoid labral and chondral injury and to permit capsulotomy closure at the end of the procedure. Thus, the interportal capsulotomy should be made 5–10 mm from the labrum and 2–4 cm long, from approximately 10 to 2 o’clock for a left hip (Fig. 2), but the interportal capsulotomy may need to be extended depending on the size of the pincer deformity. Diagnostic arthroscopy of the central compartment can then be performed safely. Acetabuloplasty rim trimming and labral treatment are performed with the hip in traction. In cases with excessive rim over-coverage or in the presence of a large hypertrophic labrum, there is greater risk of iatrogenic chondrolabral injury, and making the capsulotomy from outside in or beginning in the peripheral compartment might allow for safer entry into the central compartment under direct visualization.
Large-bore spinal needle joint entry with the bevel facing up to avoid the labrum |
Stylet removed to permit an air arthrogram image |
Stylet reinserted and the needle brought just outside of capsule |
Fluoroscopically confirm needle outside of arthrogram |
Reinsert needle back into joint with bevel facing labrum |
As soon as “pop” is felt (penetration of capsule), needle rotated 180° to avoid femoral head articular cartilage |
Stylet removed and nitinol wire placed intra-articular |
Needle removed, followed by dilation, cannulation, and arthroscope insertion |

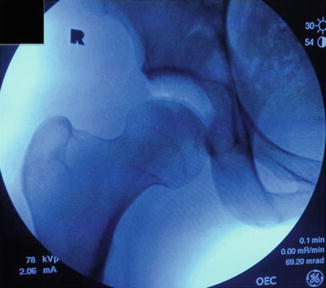
Fig. 1
Intraoperative C-arm fluoroscopy of a right hip in the supine position. A vacuum phenomenon is demonstrated after the suction seal is broken with application of traction

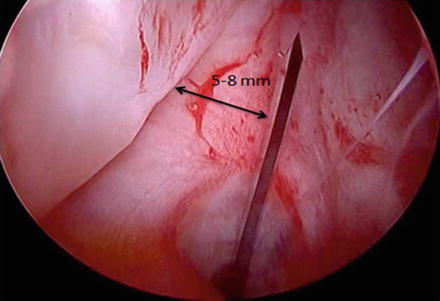
Fig. 2
Interportal capsulotomy creation may be made 5–8 mm from the acetabular labrum with an arthroscopic scalpel. This amount of acetabular side capsule permits tissue for capsular repair or plication at the conclusion of the case. The patient is in the supine position, with the right hip being viewed from the anterolateral portal
During labral refixation or reconstruction, the surgeon must be cognizant of the appropriate drill angle for anchor placement to avoid penetration through the acetabular cartilage into the joint. Using three-dimensional acetabular models of computed tomography scans of 20 cadaveric hips, the acetabular rim angle was defined and evaluated [19]. This angle quantifies the amount of acetabular bone available for drill bit and suture anchor penetration and creates a safety margin for the surgeon. Using drill bits of length between 10 and 25 mm and acetabular rim trimming amounts of 0, 2.5 and 5.0 mm, the investigation demonstrated that clock position, drill depth, and amount of rim trimming all had significant effects on the acetabular rim angle. The angle was greatest at 2 o’clock but smallest at 3 o’clock. While greater drill depth significantly reduced the rim angle, greater amounts of rim trimming significantly increased rim angle. Thus, anterosuperiorly, the surgeon must take care in drilling the minimum amount necessary to insert the anchor, especially near the 3 o’clock position. Ultimately making the portals used for placing anchors further distal typically gives a better angle for drilling and anchor placement with less risk for penetration of the acetabular articular cartilage. Beyond drilling and anchor placement, the surgeon must pass suture around or through the labrum. If suture retrieval is lost, this creates an opportunity for multiple passes of the suture-passing device through the labrum, which can potentially lead to biological disruption.
Iatrogenic Instability
There have been nine reported cases of post-arthroscopy hip dislocation [4, 20–25]. Due to publication bias, this is likely a significant underestimate of the true incidence of instability following hip arthroscopy [7]. In addition, it is likely that a number of patients have persistent disability from subtle instability postoperatively without frank dislocation which is much more difficult to define (Figs. 3 and 4). The risk of postoperative instability is related to the following patient-, hip-, and surgical technique-specific factors: type and size of capsulotomy or capsulectomy without repair, labral resection (versus refixation or repair), overaggressive rim trimming, overall capsular laxity, and psoas tenotomies [10]. Capsulotomy (interportal with or without “T” extension) permits visualization and instrumentation of the central and peripheral compartments. However, the iliofemoral ligament is the strongest of four discrete hip ligaments, and its primary purpose is to restrain external rotation and extension of the hip. This part of the capsule is vital to stability in the latter provocative positions (Fig. 5). Multiple cadaveric biomechanical studies have demonstrated that iliofemoral ligament sectioning results in increased external rotation, extension, and anterior translation [26–28]. Further, no difference exists between the repaired and intact state. Thus, unrepaired capsulotomies have the potential for postoperative instability in some situations, which falls along a spectrum of “microinstability” to frank dislocation [29–32]. Although technically demanding, there are several pearls and pitfalls to assist the surgeon in performing repair or plication (Table 4). Following open hip preservation surgery, instability has not been reported in the literature. In this situation, capsulotomies are, for the most part, repaired once the intra-articular work is complete, reducing the risk of instability. It is imperative for the surgeon to have an understanding of normal acetabular anatomy, hip dysplasia, and dysplastic variants when performing FAI corrective procedures. Excessive rim resections should be avoided in all patients, and the capsule and labrum should be repaired/preserved in borderline dysplastic hips. Psoas tenotomies should also be performed with caution as psoas impingement is frequently seen in the setting of acetabular dysplasia and excessive femoral neck anteversion, both of which can be associated with anterior hip instability. Psoas tenotomies in the presence of anterior instability can further destabilize the hip. Prior studies have reported inferior outcomes after psoas tenotomy in the presence of excessive femoral neck anteversion as well as postoperative hip dislocation after psoas tenotomy and capsulotomy performed arthroscopically [33].
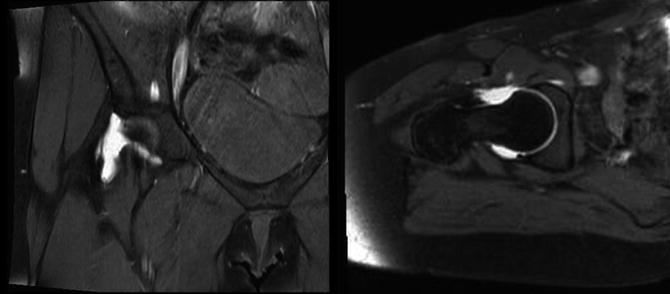
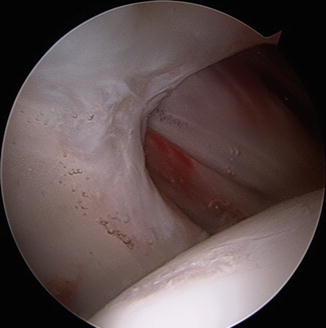
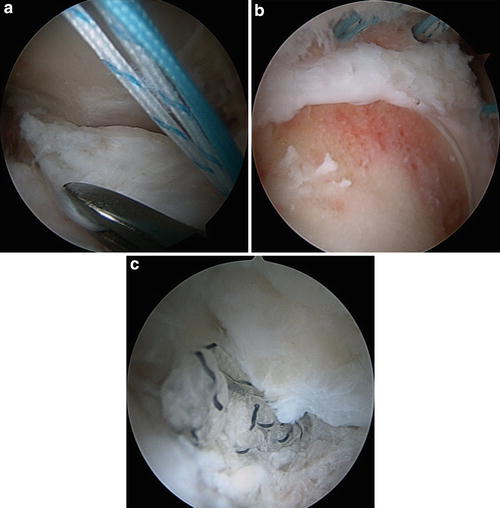

Fig. 3
MRA evidence of capsular defects after hip arthroscopy. Left: T2 coronal images demonstrating gadolinium extravasation due to capsular defect. Right: T2 axial oblique images demonstrating capsular defect with retraction of the iliofemoral ligament

Fig. 4
Arthroscopic evaluation of capsular defect involving the entire iliofemoral ligament

Fig. 5
(a) Arthroscopic view of the anterior aspect of the femoral neck. Using an arthroscopic grasper to mobilize the retracted iliofemoral ligament. (b) Arthroscopic revision femoral osteochondroplasty with three sets of double-loaded suture anchors for capsular reconstruction. (c) Arthroscopic view of the completed capsular reconstruction with suture anchors
Table 4
Pearls and pitfalls for hip capsulotomy and capsular repair or plication. DALA (distal anterolateral accessory) portal; IFL (iliofemoral ligament) [10]
Pearls | Pitfalls |
|---|---|
Interportal and “T” capsulotomy | Poor visualization |
Enhanced central and peripheral compartment visualizatio | Poor portal placement |
Refixation of labrum | Failure to address bony pathology |
Suture anchor based; as close as possible to articular margin using DALA portal | Femoral cam and acetabular pincer |
Static stability restoration | Stresses labral/capsular repair |
Femoral and acetabular osteochondroplasty | Too aggressive capsulectomy |
Reduces/eliminates impingement | Prevents complete closure or requires too much tension upon repair that predisposes to stiffness postoperatively |
Complete capsular closure | Damaged capsular edges from mechanical shaver devices may preclude secure “bite” with sutures |
Avoid aggressive capsulectomy | Avoid iatrogenic articular cartilage damage with passage of tissue penetrator/suture passer devices |
Begin closure at distal base of IFL “T’d” capsule and progress proximally toward interportal capsulotomy | Postoperative rehabilitation |
Customize degree of plication/“bite” based on patient’s ligamentous laxity status | Hip extension or external rotation that stresses capsulolabral repair, with potential disruption |
Postoperative rehabilitation | Poor patient selection |
Avoid hip extension, external rotation | Dysplasia, hyperlaxity, coxa magna |
Neurovascular Injury
Nerve or blood vessel injury is uncommon during both arthroscopic and open hip surgery [7, 9]. In hip arthroscopy, the incidence of nerve damage is 1 %, with temporary (recovery room to 4 months following surgery) neuropraxia accounting for nearly all cases. The most commonly reported affected nerve is the pudendal (40 %), followed by lateral femoral cutaneous (21 %), sciatic (17 %), common peroneal (17 %), and femoral (4.7 %) [7]. The pudendal nerve (sacral plexus; S2–S4) is both a somatic and autonomic nerve that provides sensory, motor, sympathetic, and parasympathetic function to both male and female external genitalia and sphincters of the bladder and rectum. Nerve compression between the perineal post and the inferior pubic ramus may lead to a neuropraxia, with subsequent perineal numbness, and less commonly difficulty with erection and ejaculation [34]. Urinary and/or fecal incontinence have not been reported following hip arthroscopy, likely due to the relevant innervation of the structures controlling these functions proximal to the zone of compression and the fact that bilateral nerve injury would be required in order to cause incontinence [34]. Further, inferior pubic rami anatomy is unique between genders (steep course of ramus from ischial tuberosity to pubic symphysis in males versus more rounded, gentler, and straighter course in females) [34]. Although the magnitude of traction while using a perineal post has been shown to significantly affect the incidence of pudendal nerve injury, the effect of duration of traction is less clear [35]. Additionally, a lower extremity adduction moment increases the traction force [35] and the force around the post [36]. Therefore, in order to reduce the risk of pudendal nerve compression in the perineum, the following can be helpful: general anesthesia with muscle relaxation in particular when longer traction times are required, sufficient padding of the perineal post, joint distention, and application of the least amount of traction force necessary to distract the hip sufficiently (less than 50 lb) [37] and adduction necessary to achieve joint visualization for the least amount of time. Some surgeons have even successfully performed arthroscopy without a perineal post [38].
Sciatic nerve (L4–S3) neurophysiologic monitoring during supine arthroscopy has revealed that approximately half (54 %) of subjects experience significant somatosensory evoked potential (SSEP) waveform changes [39]. These changes (signal loss) occur from 7 to 46 min from traction onset and are recovered after 2–15 min of traction release. The latter investigation’s time dependency has not been observed during lateral arthroscopy, where SSEP and also tcMEP (transcranial motor evoked potential) have been used to study this phenomenon [40]. The risk of a sciatic nerve event significantly increased 4 % with every 0.45 kg increase in traction (p = 0.043), while an increase in traction time did not significantly increase the risk of a nerve event (p = 0.201). However, only 7 % of subjects had a clinically detectable postoperative nerve injury. Historically, the 2-h time limit on duration of hip arthroscopy traction was extrapolated from the time-dependent ischemia threshold associated with tourniquet use [41]. It appears that the time dependency of neural injury is related to the perineum and pudendal nerve due to a compression or ischemic effect while in the supine position versus the magnitude dependency of neural injury which is related to the sciatic nerve and axial distraction while in the lateral position [41]. Thus, the surgeon should be mindful of both traction magnitude, especially while lateral, and traction duration, especially while supine [41].
Sufficient padding of the lower extremity in order to reduce neural compression and injury applies to the foot and ankle boot as well, not just the perineum. The superficial peroneal nerve is at risk of compression injury if the foot/ankle is improperly padded and also if the duration/magnitude of traction is excessive [37]. Femoral nerve (L2–L4) injury has also rarely been reported, secondary to either traction, fluid extravasation, or excessively medial placement of the anterior portal [7]. The lateral femoral cutaneous nerve (L2–L3) is at risk with anterior arthroscopic portal placement and open anterior approaches to the hip due to its directly subcutaneous extrafascial location. Although the nerve course is variable, it tends to pass medial to the anterior superior iliac spine (ASIS), under the inguinal ligament, on the superficial surface of sartorius muscle approximately 10–15 mm distal to the ASIS [42]. This places the nerve proper or one of its branches in very close proximity to a deep stab incision for anterior portal placement. Lateral femoral cutaneous nerve injury may cause a spectrum of symptoms ranging from benign or bothersome numbness to debilitating painful dysesthesias. Lateral femoral cutaneous neuropraxia is likely underreported, as the majority may not be noticed by the patient and found on inspection of the anterolateral thigh with nearly all resolving within 6 months post-surgery. The superior gluteal nerve limits the safe zone of the anterolateral and posterolateral portals proximally at approximately 4–7 cm [43, 44]. The sciatic nerve limits the safe zone of the posterolateral portal posteriorly at approximately 2–5 cm [43, 44]. Thus, avoidance of hip external rotation is recommended as the greater trochanter moves posteriorly blocking access for the portal and putting the nerve at greater risk.
The most common vascular structure at risk for injury anteriorly is the ascending terminal branch of the lateral femoral circumflex artery, which may be as close as 10 mm from an anterior portal [44]. However, this artery is commonly ligated during a Smith-Peterson approach just deep to the interval between the tensor fascia lata and sartorius. Due to the fact that the medial femoral circumflex is the largest contributor to the head, lateral femoral circumflex injury or ligation is likely of minimal consequence [45]. During surgical hip dislocation , when preparing to perform greater trochanteric osteotomy, it is critically important to protect the medial circumflex vessels by leaving the external rotators intact. These small muscles should remain attached to the non-osteotomized femur. When dissecting near the insertion of the external rotators on the proximal femur, the surgeon must identify the superior margin of quadratus femoris, which is marked by the trochanteric branch of the deep branch of the medial circumflex artery [45]. The trochanteric branch marks the level of the tendon of the obturator externus, which is crossed posteriorly by the deep branch of the medial circumflex. The obturator externus is responsible for protecting this vessel from either tension or compression during surgical hip dislocation. The deep branch ascends superiorly and pierces the capsule at the level of the superior gemellus. Once intracapsular, 2–4 subsynovial superior retinacular vessels pierce the head approximately 2–4 mm from the head-neck junction. Damage to the medial femoral circumflex artery or one of its branches may cause variable degrees of femoral head avascular necrosis. Following hip arthroscopy, the risk of avascular necrosis is less than 1 % (10 cases reported out of 6,334 hips) [7]. Following surgical dislocation, the risk is also significantly less than 1 % [9]. However, the intent of the surgery is to access 360° of the femoral head and acetabulum with a 0 % risk (not just less than 1 %) of avascular necrosis, implying that this complication is completely preventable with attention to appropriate technique [46]. Although avascular necrosis has been reported following surgical hip dislocation for the treatment of Perthes disease, slipped capital femoral epiphysis, and developmental hip dysplasia [47], it has not been reported for the treatment of femoroacetabular impingement. It must be recognized, however, that the surgeons performing these techniques are experienced surgeons that either developed the technique or have trained with the developers, performed hip vascular supply research with the developers, and performed a high volume of the technique. The femoral artery proper is at risk only with far medial straying of the anterior portal (3.5–4 cm medial).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








