70 Clinical Features of Rheumatoid Arthritis
Larger joints can be involved, usually later and in a symmetric fashion.
Early therapy with aggressive treatment goals improves long-term outcomes in rheumatoid arthritis.
![]() Supplemental images available on the Expert Consult Premium Edition website.
Supplemental images available on the Expert Consult Premium Edition website.
Epidemiology and the Burden of Disease
The prevalence of rheumatoid arthritis (RA) in most populations is around 1% in most populations, with an incidence in women twice that in men. This number was based on many studies of population samples,1–3 which varied among the surveys from 0.3% to 1.5%. The prevalence of RA in some populations might be changing, however, as suggested by more recent data on incidence rates in different decades. The incidence of RA in Rochester, Minnesota, decreased by 50% between 1950 and 1974. Differences between incidence and prevalence are enhanced by the realization that as the population ages, the prevalence of RA may increase or stay the same, regardless of short term trends in incidence simply because individuals with RA are living longer.
The incidence of RA increases during adulthood, except among men in their 40s through 60s. In Olmsted County, Minnesota, the increased incidence with increasing age continues until age 85, after which the incidence declines.4 In a 10-year extension of this study, the age-adjusted and sex-adjusted incidence per 100,000 population decreased from 62 in the decade 1955 to 1964 to 32.7 in the decade 1985 to 1994.5 The decrease was more prominent in women than in men, and the average age at onset of the disease shifted upward. Perhaps more intriguing were cyclic patterns of incidence within decades, suggesting the influence of environmental factors. One explanation for the decline in incidence and the shift toward older age at onset is a birth cohort effect, the greatest impact of which is seen early in life.6 A recent update from the Olmsted County, Minnesota, cohort of RA patients from 1955-2007 examined trends in the incidence and prevalence of RA from 1995-2007. During the more recent time period, the incidence of RA in women, but not in men, increased moderately.7 Causes for this trend reversal in women were not determined but might involve environmental factors. Current incidence rates using this same population-based study of Minnesota patients revealed that the lifetime risk of RA among U.S. adults is 3.6% for women and 1.7% for men.8
Throughout the world, pockets of ethnic groups have a much higher incidence of RA. Native Americans constitute one of these groups. In one geographic area between 1986 and 1994, non–Native American populations had an RA prevalence of 1.1% to 0.9%, whereas the prevalence among Algonquian Indians in the same region ranged from 2% to 2.1%, and disease onset was seen 12 years earlier in the Native American population. Among Pima Indians, who bear a very high incidence of RA, a decline in incidence has been correlated with a decrease in seropositivity for rheumatoid factor (RF). The highest likelihood of seropositivity was noted in Pima Indians born at the turn of the 20th century, and seropositivity has decreased ever since that time. This provides additional supportive evidence for a birth cohort effect.9
Although newer, more effective therapy for rheumatoid patients has led to reduced morbidity and disability from the disease, dollar costs for RA, which recently surpassed the cost per patient for diabetes, are still substantial. In a panel of individuals with RA in San Francisco followed for 15 years, medical care costs for RA averaged $5,919 per year, and additional costs of $2,582 were incurred for medical but non-RA reasons.10 More than half of these costs were generated by hospitalization, with some patients bearing costs greater than $85,000/year while their function declined. In another cohort of 4258 patients with RA followed for 17,085 patient-years, lifetime direct medical care costs were estimated to be $93,296.11
Risk Factors
A predisposition to RA appears to be multifactorial based on the following: (1) Relatively few identical twins have RA (about 15%), even though concordance for the disease is much more likely in twins than in the normal population; (2) despite the powerful influence of the “shared epitope” on HLA-DRB chains in predisposing to the severity of disease, this susceptibility allele is not a risk factor in certain population studies; and (3) the combination of many gene polymorphisms confers a modestly increased risk for disease. A reasonable hypothesis is that the genetic predisposition to RA involves a propensity to autoimmune responses, but that repeated exposure to environmental agents is ultimately responsible for tipping the balance from subclinical autoimmunity to diseases such as RA. Many of the risk factors for RA are discussed in Chapter 69, especially genetic associations, environmental exposures, and the role of autoantibodies.
Clinical Presentations of Early Rheumatoid Arthritis
In the Northern hemisphere, the onset of RA is more frequent in winter than in summer. In several series, onset of RA from October to March in the Northern hemisphere was found to be twice as frequent as in the other 6 months.12 The appearance of RF or anticitrullinated protein antibodies (ACPAs), also referred to as anticyclic citrullinated protein antibodies (anti-CCPs), often precedes symptoms of arthritis in patients. Approximately half of patients with RA have specific serologic abnormalities several years before the onset of symptoms. A finding of an elevated serum level of immunoglobulin (Ig)M-RF or anti-CCP in a healthy person correlates with increased risk of developing RA.13 This is especially important in light of the new criteria for RA classification in early RA because symptoms can be minimal for some of those who meet the criteria for diagnosis (see later).
Patterns of Onset
Insidious Onset
RA has an insidious, slow onset over weeks to months in 55% to 65% of cases.14 The initial symptoms may be systemic or articular. In some people, fatigue, malaise, swollen hands, and diffuse musculoskeletal pain may be the first nonspecific symptoms, with joints becoming involved later. Involvement of tendon sheaths early in the process can focus attention on periarticular structures. In retrospect, the patient often can identify one joint that was involved first, quickly followed by the others. Asymmetric initial presentations (often with increased symmetry developing later in the course of disease) are common. The reason for the symmetry of joint involvement compared with other forms of arthritis, such as the seronegative spondyloarthropathies, is unknown.
Joint Involvement
The joints most commonly involved first in RA are the metacarpophalangeal (MCP) joints, the proximal interphalangeal (PIP) joints, the metatarsophalangeal joints, and the wrists (Table 70-1).15 Larger joints generally become symptomatic after small joints. Synovitis in large joints is likely to remain asymptomatic for a longer time than in smaller ones, and a biopsy specimen of an asymptomatic knee often shows histologic evidence of synovitis.16 One anatomic study correlated the area, in square centimeters, of synovial membrane with that of hyaline cartilage in each joint. Joints with the highest ratio of synovium to articular cartilage correlated positively with those most frequently involved in the disease (see Table 70-1).17
Table 70-1 Distribution of Joints Involved in Attacks Based on Cumulative Experience with 227 Patients
| Joint Involvement | % Patients (Mean) | % Patients (Range) |
|---|---|---|
| MCP, PIP | 91 | 74-100 |
| Wrists | 78 | 54-82 |
| Knees | 64 | 41-94 |
| Shoulders | 65 | 33-75 |
| Ankles | 50 | 10-67 |
| Feet | 43 | 15-73 |
| Elbows | 38 | 13-60 |
| Hips | 17 | 0-40 |
| Temporomandibular | 8 | 0-28 |
| Spine | 4 | 0-11 |
| Sternoclavicular | 2 | 0-6 |
| Peri-articular sites | 27 | 20-29 |
MCP, metacarpophalangeal; PIP, proximal interphalangeal.
Modified from Guerne P-A, Weisman MH: Palindromic rheumatism: part of or apart from the spectrum of rheumatoid arthritis, Am J Med 16:451-460, 1992. Copyright 1992, with permission from Excerpta Medica, Inc.
Early Synovitis: Which Patients Develop Rheumatoid Arthritis?
Some of these questions have been addressed by the Leiden Early Arthritis Clinic, which evaluates patients with symptoms of less than 2 years’ duration (most patients have symptoms for less than 6 months). In this cohort, only about 20% of patients met criteria for RA when initially evaluated by a rheumatologist.18 One-third of patients defied categorization and were considered to have “undifferentiated arthritis.” When this group of patients was followed for 1 year, 27% ultimately developed RA, and 40% remained undifferentiated. Clinical features that were more commonly seen among patients who developed RA included greater numbers of joints involved (mean of seven joints vs. four joints), longer duration of morning stiffness (90 minutes vs. 60 minutes), and the presence of autoantibodies. These features were insufficient individually to permit early diagnosis of RA, although a composite scoring system has been proposed.19 The predictive value of this system approaches 90%.
Additional studies from the Leiden Early Arthritis Clinic show that assessment and management of patients with symptoms of less than 12 weeks’ duration by a rheumatologist was associated with decreased joint destruction and increased likelihood of disease-modifying antirheumatic drug (DMARD)-free remission.20 Treatment of early inflammatory synovitis with methotrexate therapy delays progression to RA, but disease ultimately progresses to RA if the medication is discontinued21 (see Chapter 42).
Among predictive features most likely to be useful in patients, serum autoantibodies might be the most important. ACPAs, in particular, are strongly associated with the evolution of undifferentiated arthritis into RA and progression to erosive disease. In addition, the diversity of citrullinated peptides recognized by ACPAs increased during the period preceding onset of disease in patients who progressed to RA, suggesting that epitope spreading plays a role in the evolution of disease.22 Other autoantibodies have also been used in a diagnostic algorithm for patients with very early synovitis (symptoms of <3 months’ duration), including RFs, anticitrullinated protein, and anti-RA33.23 Through stepwise analysis of each antibody, RA could be diagnosed in 72% of patients and confirmed by subsequent clinical course and development of RA.
Other Patterns of Disease Onset or Variants of Disease
Palindromic Pattern
Palindromic rheumatism was described by Hench and Rosenberg in 1942. Pain usually begins with pain in one joint or in periarticular tissues; symptoms worsen for several hours to a few days and are associated with swelling and erythema. Then, in reverse sequence, symptoms resolve, leaving no residua. Table 70-2 lists joints involved in a series of 227 patients. An intercritical period, similar to that of gout, is asymptomatic. Half of patients with palindromic rheumatism go on to develop RA, particularly those with HLA-DR4. In a compilation of patients from nine series, only 15% became asymptomatic after at least 5 years with a palindromic syndrome (see Table 70-2).24 In the remainder, multiple joints became involved, swelling did not subside completely between attacks, and tests for RF became positive. Neither the characteristics of joint fluid nor the pathologic findings of synovial biopsy specimens allow the prediction that RA will evolve from palindromic rheumatism,25 although it may be worthwhile to measure ACPAs in these people.26 Those who do not develop RA rarely have constitutional symptoms, and involved joints show no erosion because the synovitis does not become chronic. A more recent study of long-term outcomes in 60 patients diagnosed with palindromic rheumatism revealed that two-thirds of patients developed chronic arthritis, and the risk for chronic arthritis remained for longer than 10 years.27 Of 51 patients with palindromic rheumatism, 41 experienced marked improvement in frequency and duration of attacks during treatment with antimalarials.28 The use of antimalarials might reduce the risk of progression to RA.
Insidious Onset in Older Individuals
In a study of patients with RA of less than 15 months’ duration, older patients had higher scores for joint space narrowing and osteophytes at baseline than patients younger than 55 years. However, no evidence suggested that older patients had more rapid progression of damage, indicating that osteoarthritis was responsible for a significant portion of the damage noted at the onset of disease.29
Arthritis Robustus
Arthritis robustus is not so much an unusual presentation of disease as an unusual reaction of patients to the disease.30 Most patients are men whose disease is characterized by proliferative synovitis, often with deformity, which seems to cause little pain and even less disability. Patients are athletic and invariably keep working (often at physical labor). Periarticular osteopenia is unusual, whereas new bone proliferation at joint margins near significant erosions of bone and cartilage is common. Bulky subcutaneous nodules develop. Subchondral cysts also develop, presumably from excessive pressure caused by synovial fluid within a thick joint capsule during muscular effort.
Rheumatoid Nodulosis
Whether rheumatoid nodulosis is a variant subset of RA or a different entity has not been clarified. The clinical picture includes recurrent pain and swelling in different joints, radiologic subchondral bone cysts, and subcutaneous rheumatoid nodules. In one series of 16 patients followed over 12 years, 6 had an aggressive course indistinguishable from classic erosive polyarticular RA. In 7 patients, cholesterol crystals were found in fluid from the olecranon bursae. Second-line drugs helped articular disease but did not help other components of the process.31
Course and Complications of Established Rheumatoid Arthritis
Involvement of Specific Joints: Effects of Disease on Form and Function
Hands and Wrists
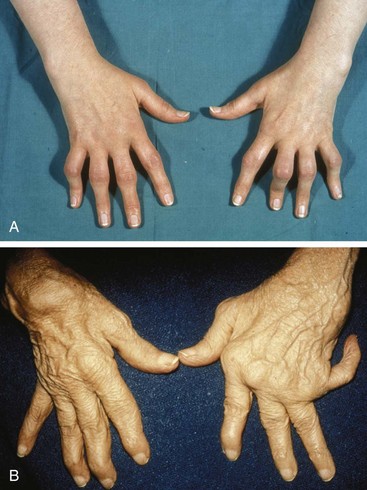
The hand and the wrist should be considered together because they form a functional unit. Data have linked disease of the wrist to ulnar deviation of the MCP joints.32 The hypothesis is that weakening of the extensor carpi ulnaris muscle leads to radial deviation of the wrist as the carpal bones rotate (the proximal row in an ulnar direction and the distal ones in a radial direction). In response to this, ulnar deviation of the fingers (a “zigzag” deformity) keeps the tendons to the phalanges in a normal line with the radius. Other factors, including the tendency for a power grasp to pull the fingers into an ulnar attitude and inappropriate intrinsic muscle action, are also involved (Figure 70-1). Erosion of bone or articular cartilage is not essential for the development of ulnar deviation (Figure 70-2). Significant but reducible ulnar deviation can result from repeated synovitis or muscle weakness in the hands (e.g., in systemic lupus erythematosus, in Parkinson’s disease).
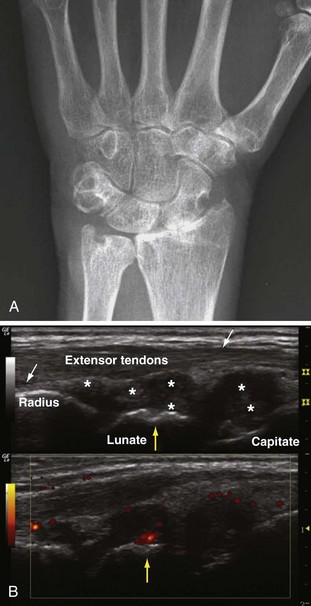
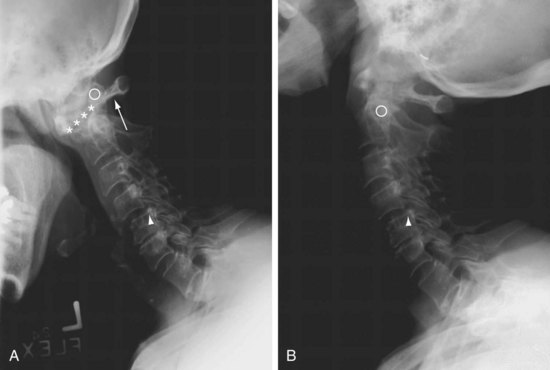
Progression of disease in the wrist may be characterized by radiographic loss of joint space and bone or by ankylosis (Figure 70-3A). Ultrasound of the wrist correlates with function and classic signs of inflammation and is a complementary tool in the evaluation of wrist arthritis in RA (Figure 70-3B). Early detection of carpal bone involvement by RA is also possible with magnetic resonance imaging (MRI), which reveals early synovial proliferation and carpal bone erosions. Bony ankylosis is associated with increased duration and severity of the disease and is found in joints that have been immobilized by pain, inflammation, treatment, or all of these.
The hand may have many joints involved in RA. A sensitive index of hand involvement is grip strength, which simulatneously stresses multiple hand joints. Muscular contraction causes ligamentous tightening around joints, compressing inflamed synovium. The immediate result is weakness, with or without pain; the reflex inhibition of muscular contraction due to pain may be a primary factor in this weakness. Quantitative radiographic scores for joint space narrowing, erosion, and malalignment correlate well with loss of motion but do not correlate with joint count tenderness scores33; these data support the concept that inflammatory synovitis and the erosive-destructive potential of proliferative synovitis in RA are not one and the same, but rather reflect different aspects of the same disease.
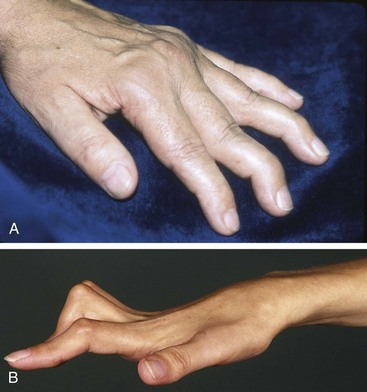
The swan neck deformity is one of flexion of the distal interphalangeal (DIP) and MCP joints with hyperextension of the PIP joint. The lesion probably begins with shortening of the interosseous muscles and tendons. Shortening of the intrinsic muscles exerts tension on the dorsal tendon sheath, leading to hyperextension of the PIP joint (Figure 70-4A).34 Deep tendon contracture or, rarely, DIP joint involvement with RA leads to the DIP joint flexion. Marginal erosive changes in DIP joints occur more often in patients with RA who have coexisting osteoarthritis.35
If, during chronic inflammation of a PIP joint, the extensor hood stretches or is avulsed, the joint may pop up in flexion, producing a boutonnière deformity (Figure 70-4B). The DIP joint remains in hyperextension.
Three types of deformity have been described for the thumb:
1. MCP inflammation leads to stretching of the joint capsule and a boutonnière-like deformity.
2. Inflammation of the carpometacarpal joint leads to volar subluxation during contracture of the adductor hallucis.
3. After prolonged disease of both MCP joints, exaggerated adduction of the first metacarpus, flexion of the MCP joint, and hyperextension of the DIP joint result from the patient’s need to provide a means to pinch.
One of the most common manifestations of RA in the hands is tenosynovitis in flexor tendon sheaths; this can be a major cause of hand weakness.36 Tenosynovitis manifests on the volar surfaces of the phalanges as diffuse swelling between joints or as a palpable grating within flexor tendon sheaths in the palm and may occur in half of RA patients.
Shoulders
RA of the shoulder not only affects synovium within the glenohumeral joint but also involves the distal third of the clavicle, various bursae and the rotator cuff, and multiple muscles around the neck and chest wall. Severe shoulder pain is often bilateral and can lead to sleep disorders because of difficulty finding a comfortable position. Involvement of the rotator cuff in RA also has been recognized as a principal cause of morbidity. The function of the rotator cuff is to stabilize the humeral head in the glenoid. Weakness of the cuff results in superior subluxation. Rotator cuff tears or insufficiency from other causes can be shown by shoulder arthrography or MRI. In a series of 200 consecutive patients with RA studied by arthrography, 21% had rotator cuff tears, and an additional 24% had evidence of frayed tendons.37 One likely mechanism behind tears is that the rotator cuff tendon insertion into the greater tuberosity is vulnerable to erosion by the proliferative synovitis that develops there. Previous injury and aging may predispose to the development of tears. Sudden tears may be accompanied by pain and inflammation so great as to suggest infection.
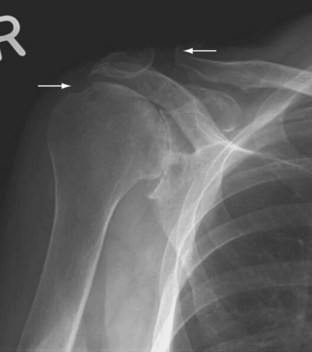
Standard radiographic examinations of the shoulder in RA reveal erosions and superior subluxation (Figure 70-5). Arthrograms, in addition to showing tears of the rotator cuff, can show diffuse nodular filling defects, irregular capsular attachment, bursal filling defects, adhesive capsulitis, and dilation of the biceps tendon sheath (perhaps unique to RA).38 High-resolution computed tomography (CT) or MRI may provide much of this information without the need for invasive techniques.
Temporomandibular Joints
Temporomandibular joint abnormalities are common in nonrheumatoid populations. The only specific findings for RA in the temporomandibular joint are erosions and cysts of the mandibular condyle detected by CT or MRI. No correlation has been noted between clinical and CT findings of the temporomandibular joint in RA.39
Cricoarytenoid Joints
The cricoarytenoid joints are small diarthrodial joints with an important function: They rotate with the vocal cords as the vocal cords abduct and adduct to vary the pitch and tone of the voice. Careful histories may reveal hoarseness in 30% of rheumatoid patients. This hoarseness is not disabling in itself, but the cricoarytenoid joints may become inflamed and immobilized, with the vocal cords adducted to the midline, causing inspiratory stridor. Autopsy examinations have shown cricoarytenoid arthritis in almost half of patients with RA, suggesting that much significant disease of the larynx may be asymptomatic. Although CT scans detected laryngeal abnormalities in 54% of patients with moderately severe RA, no symptoms suggested that these abnormalities would be found.40 In contrast, findings on indirect laryngoscopy, which detected mucosal and gross functional abnormalities (including rheumatoid nodules), were abnormal in 32% of the same patients and correlated with symptoms of sore throat and difficult inspiration. It follows that the latter examination should be performed in symptomatic rheumatoid patients. Asymptomatic cricoarytenoid synovitis occasionally may lead to aspiration of pharyngeal contents, particularly at night.
Cervical Spine
In contrast to other nonsynovial joints, such as the manubriosternal joint or the symphysis pubis, diskovertebral joints in the cervical spine often manifest osteochondral destruction in RA and on lateral radiographs may be found to be narrowed (Figure 70-6). Significant pain is reported, but passive range of motion in the absence of muscle spasm may be normal. At least two possible mechanisms have been put forth for this process: (1) extension of the inflammatory process from adjacent neurocentral joints (the joints of Luschka), which are lined by synovium, into the discovertebral area, and (2) chronic cervical instability initiated by apophyseal joint destruction leading to vertebral malalignment or subluxation. This process may produce microfractures of the vertebral end plates, disk herniation, and degeneration of disk cartilage. The atlantoaxial joint is prone to subluxation in several directions and is summarized later.
• The atlas can move anteriorly on the axis (most common). This results from laxity of the ligaments induced by the development of proliferative synovial tissue in adjacent synovial bursae or by fracture or erosion of the odontoid process.
• The atlas can move posteriorly on the axis. This can occur only if the odontoid peg has been fractured from the axis or destroyed.
• The atlas can sublux vertically in relation to the axis (least common). This results from destruction of the lateral atlantoaxial joints or of bone around the foramen magnum. Vertical (superior) migration of the odontoid can develop from unattended anterior or posterior subluxation.
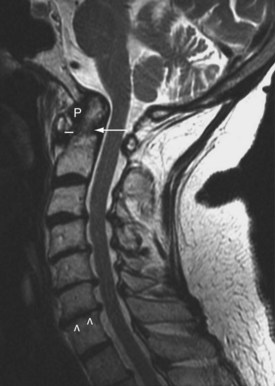
Physical findings suggestive of atlantoaxial subluxation include loss of occipitocervical lordosis, resistance to passive spine motion, and abnormal protrusion of the axial arch felt by the examining finger on the posterior pharyngeal wall. Radiographic views (lateral, with the neck in flexion) reveal more than 3 mm of separation between the odontoid peg and the axial arch. In symptomatic patients, films in flexion should be taken only after radiographs (including an open-mouth posteroanterior view) have ruled out an odontoid fracture or severe atlantoaxial subluxation. Studies have indicated that CT is useful for showing spinal cord compression by loss of posterior subarachnoid space in patients with C1 to C2 subluxation. MRI has proved particularly valuable in determining pathologic anatomy in this syndrome (Figure 70-7).
The progression of peripheral joint erosions parallels cervical spine disease in RA. The two coincide in severity and timing; cervical subluxation is more likely to develop in patients with erosion of the hands and feet. In a series of patients with RA referred for hip or knee arthroplasty, 61% had radiographic evidence of cervical spine instability.41
Is mortality increased in patients with atlantoaxial subluxation? Neurologic signs do not inevitably develop in patients with large subluxations. When signs of cervical cord compression do appear, however, myelopathy progresses rapidly, and 50% of patients die within 1 year.42 These patients are at risk for these complications if they sustain small falls, whiplash injuries, and general anesthesia with intubation. Cervical collars can be prescribed for symptomatic relief. Operative stabilization may be considered if symptoms are progressive.
Some data support the hypothesis that early C1-to-C2 fusion for atlantoaxial subluxation before the development of superior migration of the odontoid decreases the risk for further progression of cervical spine instability.43 The incidence of sustained neurologic deterioration related to surgery may be 6%; this emphasizes the importance of a skilled surgical team and of careful assessment of each patient. In many cases, surgical intervention in asymptomatic patients is riskier than conservative management despite the dire appearance of imaging studies.
Hips
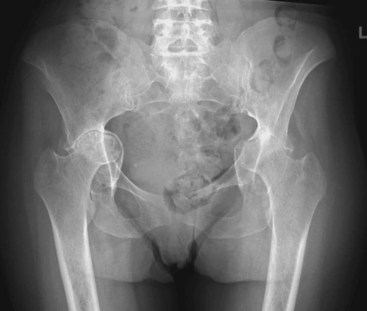
About half of patients with well-established RA have radiographic evidence of hip disease. In contrast to osteoarthritis, in which the femoral head usually migrates superiorly, symmetric thinning of the cartilage in RA leads to axial migration. The femoral head may collapse and be reabsorbed, and the acetabulum is remodeled and pushed medially, leading to protrusio acetabuli (Figure 70-8). Significant protrusion occurs in about 5% of all patients with RA.44 Loss of internal rotation on physical examination correlates best with radiographic findings. Similar to the situation in other weight-bearing joints, the femoral head may develop cystic lesions that communicate with the joint space.
Knees
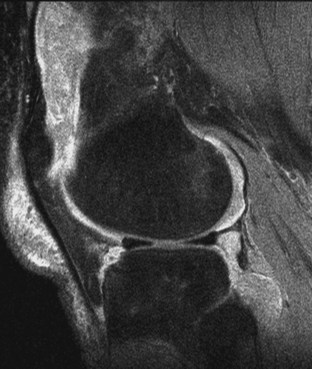
Flexion of a knee with a moderate to large effusion markedly increases intra-articular pressure. The increased intra-articular pressure may cause an outpouching of posterior components of the joint, producing a popliteal or Baker’s cyst. This can generate pressures so high in the popliteal space that it may rupture down or dissect into the calf or, less often, superiorly into the posterior thigh. Rupture occurs posteriorly between the medial head of the gastrocnemius and the tendinous insertion of the biceps. Clinically, popliteal cysts and their complications have several manifestations. An intact popliteal cyst may compress superficial venous flow from the lower leg, producing dilation of superficial veins, edema, or both.45 Rupture of the joint posteriorly with extravasation of joint fluid into the calf may resemble acute thrombophlebitis with swelling and tenderness and may produce systemic signs of fever with leukocytosis. One helpful sign in identifying cyst rupture may be the appearance of a crescentic hematoma beneath one of the malleoli of the ankle.46 Although arthrography clearly defines the abnormal anatomy of a Baker’s cyst, this invasive procedure has been replaced by ultrasonography and, when necessary, MRI (Figure 70-9).
Ankles and Feet
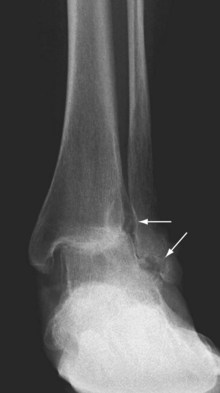
Ankle involvement is usually mild in RA, but damage can occur in severe progressive forms of the disease. Clinical evidence for ankle involvement consists of cystic swelling anterior and posterior to the malleoli. Much of the stability of the ankle depends on the integrity of the ligaments holding the fibula to the tibia and these two bones to the talus. In RA, inflammatory and proliferative disease may loosen these connections by stretching and eroding the collagenous ligaments, causing erosions (Figure 70-10). The result is incongruity, which progresses to pronation deformities and eversion of the foot.
The Achilles tendon is a major structural component and kinetic force in the foot and ankle. Rheumatoid nodules develop in this collagenous structure, and spontaneous rupture of the tendon has been reported when diffuse granulomatous inflammation is present.47 The subtalar joint controls eversion and inversion of the foot on the talus; patients with RA invariably have more pain while walking on uneven ground; this is related to the relatively common subtalar joint involvement in RA. Progressive eversion at the subtalar joint, combined with foot pain, leads to a lateral subluxation beginning in the midfoot and the development of a rocker-bottom deformity. Midfoot disease leads to collapse of the arch, which contributes to difficulty walking because of pain.
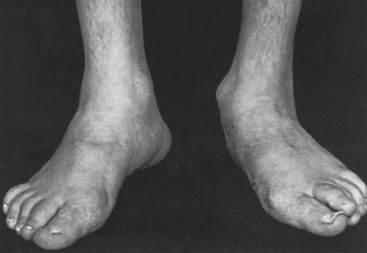
More than one-third of patients with RA have significant disease in the feet (Figure 70-11). Metatarsophalangeal (MTP) joints are often involved, and gait is altered as pain develops during push-off in striding. Studies have shown that MTPs are the initial site of erosion in many patients. Downward subluxation of the metatarsal heads occurs soon after the MTP joints become involved, producing “cock-up” toe deformities of the PIP joints. Hallux valgus and bunion or callus formation occur if disease continues. Cystic collections representing outpouchings of flexor tendon sheaths often develop under the MTP joints.48 Patients with subluxation of metatarsal heads can develop pressure necrosis of the plantar surfaces. Alternatively, those who have subluxation of MTP joints often develop ulceration over the PIP joints that protrude dorsally (hammer toes). The net result is increased pressure on the MTP joints with a sensation described as “walking on marbles” by many patients. Changes caused by the progress of disease and destruction in the foot include intermetatarsal joint ligament stretching in response to inflammation, spreading of the forefoot, anterior migration of the plantar fat pad, and dorsal subluxation of toes followed by plantar subluxation of the metatarsal heads.49 Concurrently, hallux valgus results in progressive overlap of the second and third toes on top of the great toe.
Extra-articular Complications of Rheumatoid Arthritis
Generally, the number and severity of extra-articular features vary with the duration and severity of the disease. Several of these features may be related to extra-articular foci of an immune response,50 based on evidence of independent and qualitatively different production of RF in the pleural space, pericardium, muscle, and even meninges. Patients with systemic immune responses have true rheumatoid disease, not just RA. Other unusual proteins and protein complexes in the circulation of patients with active rheumatoid disease include antiphospholipid antibodies, circulating immune complexes, and cryoglobulins. Extra-articular manifestations of RA are associated with excess mortality.51
Rheumatoid Nodules
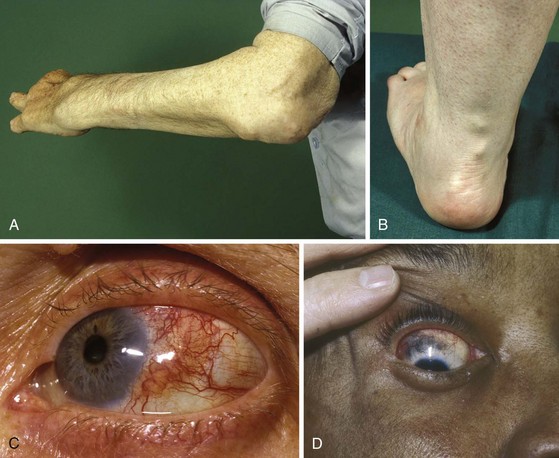
The mature rheumatoid nodule has a central area of necrosis rimmed by a corona of palisading fibroblasts that is surrounded in turn by a collagenous capsule with perivascular collections of chronic inflammatory cells. The earliest nodules, nests of granulation tissue, have been identified measuring less than 4 mm. These nodules grow by accumulating cells that expand centrifugally, leaving behind central necrosis initiated by vasculopathy and compounded by protease destruction of the connective tissue matrix. Nodules occur in 15% to 20% of patients with definite arthritis or RA. They occur most often on extensor surfaces or pressure points, such as the olecranon process and the proximal ulna (Figure 70-12A), as well as on tendons (Figure 70-12B). They are subcutaneous and vary in consistency from a soft, amorphous, entirely mobile mass to a hard, rubbery mass attached firmly to the periosteum.
The appearance of nodules in unusual sites can lead to confusion in diagnosis; they sometimes can appear identical to other types of nodules such as tophi. Sacral nodules may be mistaken for bedsores if the overlying skin breaks down. Occipital nodules also occur in bedridden patients. In the larynx, rheumatoid nodules on the vocal cords may cause progressive hoarseness. Nodules can even be found in the heart and lungs (see later). Nodules on the sclera can produce perforation of this collagenous tissue. Multiple reports describe rheumatoid nodule formation within the central nervous system, involving leptomeninges more frequently than parenchyma.52 Some patients develop rheumatoid nodules within vertebral bodies, resulting in bone destruction and signs of myelopathy.
Careful histologic study of early lesions53 suggests that development of the nodule is mediated by affected small arterioles and resulting complement activation and terminal vasculitis. This immunologic response is linked to proliferation of resident histiocytes and fibroblasts and to an influx of macrophages from the circulation. Proliferation of cells and the supporting scaffold of connective tissue are mediated by cytokines expressed in patterns similar to those found in rheumatoid synovium. The cytokine profile of the rheumatoid nodule suggests a T helper-1 (Th1) gene expression pattern similar to that of synovial tissue, including expression of tumor necrosis factor (TNF), interleukin (IL)-1, IL-12, and interferon (IFN)-γ, but not IL-2. More recently, gene expression of IL-17 family members in rheumatoid nodules showed that IL-17A gene expression was minimal in contrast to that of rheumatoid synovial tissue.54 The absence of IL-17A could be due to decreased IL-23 expression in the nodule. Data from studies using monoclonal antibodies against receptors for complement C3b and C3bi, monocytes, activated macrophages, and HLA-DR molecules suggest that mononuclear phagocytes are constantly being recruited into peripheral layers and subsequently migrate into the palisade to constitute most of the cell population in this area.55 Other studies using cytochemical markers (nonspecific esterase and CD68 for macrophages, and prolyl hydroxylase for fibroblasts) indicate that a mixture of macrophages and fibroblasts makes up the cellular content of nodules.56 This evidence fits with data from nodule tissue in organ culture; similar to synovial tissue, cells in the palisading region have the capacity to produce collagenase and protease in large quantities.57
RF is almost always found in the serum of patients with rheumatoid nodules. Rarely, such nodules are present in the absence of obvious arthritis. A condition called rheumatoid nodulosis is characterized by the presence of multiple nodules on the hands, a positive test for RF, episodes of acute intermittent synovitis, and subchondral cystic lesions of small bones of the hands and feet.58 Many clinicians have noted that during methotrexate therapy, existing nodules may enlarge and new ones may develop, even though symptoms of synovitis regress; the pathophysiology underlying this phenomenon is unknown, although it may relate to the effects of methotrexate on adenosine (see Chapter 61). Discontinuing methotrexate in these patients usually leads to regression of some nodules. Some case reports suggest that TNF inhibitors can be associated with accelerated rheumatoid nodulosis.
Bone Density
Because postmenopausal women are at greater risk for osteoporosis, this group should be treated aggressively. Minimizing steroid use is one method that can be used to decrease the risk of osteoporosis in this group and in other patients with RA. Two-phase loss of bone seems to be induced by glucocorticoids: a rapid first phase, when 12% of bone mass disappears in the first 6 to 12 months of therapy, followed by a subsequent chronic phase, which has a slower rate of bone loss.59 It is encouraging that axial bone loss in patients with RA induced early by glucocorticoids can be reversed.60 The evaluation, biology, and management of osteoporosis are discussed in Chapter 101. In the relationship between RA and bone, the focus is, appropriately, on osteoporosis; however, diffuse loss of bone in RA, whether or not it is related to glucocorticoid therapy, leads to a high incidence of stress fractures of long bones in RA.61 The fibula is the most common fracture site. Acute leg pain in a thin, elderly rheumatoid patient, even without a history of trauma, should generate suspicion of a stress fracture. Geodes (i.e., subchondral cysts developed by synovial penetration of the cortex or subchondral plate and subsequent proliferation) weaken bone and can predispose bone to fracture.
Muscle
In an early autopsy series, focal accumulations of lymphocytes and plasma cells with some contiguous degeneration of muscle fibers were found in all rheumatoid patients—a condition termed nodular myositis. More recent studies have pointed to multiple types of muscle disease in RA, although clinically relevant active myositis is uncommon62:
• Diminution of muscle bulk with atrophy of type II fibers
• Peripheral neuromyopathy, usually due to a mononeuritis multiplex
• Active myositis and muscle necrosis with foci of endomysial mononuclear cell infiltration
• Chronic myopathy resembling a dystrophic process, probably the end stage of inflammatory myositis
Skin
The most frequently recognized skin lesion in RA is the rheumatoid nodule but several other manifestations may be observed as well. “Senile” purpura resulting from skin atrophy and capillary fragility is especially common in patients treated with glucocorticoids. Palmar erythema is common, but Raynaud’s syndrome is rare. Manifestations of vasculitis range from occasional nail fold infarcts to a deep, erosive, scarring pyoderma gangrenosum. Palpable purpura in rheumatoid patients often occurs as a reaction to a drug that the patient is taking but can be primary and a direct function of the severity of articular disease. Livedo reticularis, the lacy, dusky purple, asymptomatic discoloration seen on the extremities, is believed to signify a deep dermal vasculopathy. It can be present in any or all diffuse connective tissue diseases and often is associated with antiphospholipid antibodies.63
Eye
Virtually all ocular manifestations of RA can be considered complications of the disease (see Chapter 44). Keratoconjunctivitis sicca, a component of Sjögren’s syndrome, is discussed in Chapter 73. Scleritis and episcleritis are associated with RA. Highly differentiated connective tissues in the eye make rheumatoid manifestations particularly interesting and, when they occur in aggressive form, very serious.
The episclera of the eye is highly vascular compared with the dense sclera. Scleritis, episcleritis, or both occur in less than 1% of rheumatoid patients. In episcleritis, the eye becomes red and, in contrast to conjunctivitis, causes tearing but no discharge. Loss of vision does not occur as a direct result of episcleritis, but a keratitis or a cataract developing secondarily can cause visual loss. Scleritis causes severe ocular pain and dark red discoloration (Figure 70-12C). No discharge is present. Depending on the intensity of the process, scleritis can be localized and superficial or generalized, with or without granulomatous resorption of the sclera down to the uveal layer; when this complication occurs, it is termed scleromalacia perforans. In contrast to superficial eye disease, which usually can be treated conservatively with topical steroids, scleritis usually requires systemic or intraocular corticosteroid treatment. In some cases, the sclera can become thin even in the absence of overt inflammation, leading to scleromalacia (Figure 70-12D). Rarely, perilimbic ischemic ulcers can be caused by cryoproteins (RF-IgG complexes) and if untreated can result in perforation of the anterior chamber. Patients with RA who have an associated keratoconjunctivitis sicca secondary to Sjögren’s syndrome have pruritic and painful eyes, sometimes leading to chronic blepharitis.
Host Defense and Infection
The incidence of infection as a complication of RA has paralleled the use of glucocorticoids, biologics, and immunosuppressive agents. TNF blockers are especially noteworthy because they have been associated with reactivation of tuberculosis and other opportunistic infections such as histoplasmosis. Pulmonary infections, skin infections, and septic arthritis are the most common infections in RA.64,65 Difficulty in diagnosis is accentuated by the similarity of aggressive RA to infection, particularly in joints; a “pseudoseptic” arthritis in rheumatoid patients, associated with fever, chills, and grossly purulent synovial fluid, can be part of a severe exacerbation of RA and must be distinguished from infection.66 Mortality attributable to respiratory infections such as pneumonia and bronchitis is increased in RA patients compared with the general population.67 A retrospective longitudinal cohort study compared the frequency of infection in a population-based incidence cohort of RA patients versus that in a group of individuals without RA from the same population; this study looked at 7900 to 9100 person-years.68 A total of 609 RA patients and 609 non-RA patients were studied; 73% were women, and mean patient age was 58 years. Hazard ratios for RA patients versus controls after adjustment for age, sex, smoking status, leukopenia, corticosteroid use, and diabetes mellitus were nearly twofold increased for confirmed infection and for infection requiring hospitalization. Bone, joints, skin, respiratory tract, and soft tissues were the organs with highest hazard ratios. In a subsequent study in this cohort, predictors of infection were identified as increasing age, extra-articular manifestations of RA, leukopenia, and comorbidities such as chronic lung disease, alcoholism, diabetes mellitus, and the use of glucocorticoids.
Traditional DMARD use generally is not associated with a major increased incidence of infection. However, vigilance in using biologic agents is essential because mortality is increased from infection due to immunosuppression.69 Physicians should always have a low threshold of concern for infection in rheumatoid patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree