37 Cancer Risk in Rheumatic Diseases
Accelerated growth of cancer cells in immunodeficient mice and increased risk of cancer in heavily immunosuppressed transplant patients have shaped the perception of the immune system as a potent barrier against neoplasms.1,2 It might be expected that immunosuppressive treatment would inevitably result in effects favoring malignant cell growth. However, emerging evidence supports the seemingly paradoxical notion perhaps formulated first by Rudolph Virchow in 1863 that inflammation is a critical component of cancer initiation and progression, and that reduction of systemic inflammation may reduce cancer risk in these conditions.3
Assessment of cancer risk in rheumatic diseases must be weighed against the lifetime risk of developing cancer, which is approximately 20% in Western Europe and North America, with 5% of the general population having current cancer or a history of cancer.4 Approximately 1 in 10 women will develop breast cancer, and as many as 1 in 8 men will develop prostate cancer, 1 in 25 colorectal cancer, 1 in 40 lung cancer, and approximately 1 in 100 lymphoma or other lymphoproliferative malignancy.4
The combination of increased risk for some and decreased risk for other types of cancers in different rheumatic diseases may result in a neutral effect for malignancies in general, emphasizing why, from a clinical standpoint, it is important to identify risks pertaining to specific cancers, which may be uncommon. The statistical approach for capturing differences in sparse event data, particularly when malignancy is not a prespecified study outcome, and assumptions of proportional hazards models and stable frequencies of events over time for a nonlinear risk such as cancer can lead to major errors in interpretation.5
Malignancy in Autoimmune Rheumatic Diseases
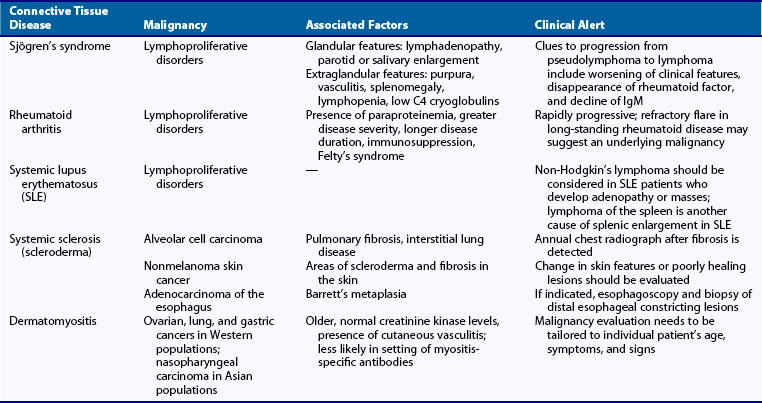
Several of the rheumatic diseases, particularly lymphoproliferative disorders, appear to be associated with increased risk of malignancy. A list of rheumatic diseases that have been associated with malignancy is provided in Table 37-1. In a global assessment of susceptibility to Hodgkin’s lymphoma using population-based linked registry data from Sweden and Denmark, 32 autoimmune and related conditions were identified from hospital diagnoses in 7476 case subjects with Hodgkin’s lymphoma, 18,573 matched control subjects, and more than 86,000 first-degree relatives of case and control subjects. Significantly increased risks of Hodgkin’s lymphoma were associated with a personal history of autoimmune disorders, including rheumatoid arthritis (odds ratio [OR], 2.7; 95% confidence interval [CI], 1.9 to 4.0), systemic lupus erythematosus (OR, 5.8; 95% CI, 2.2 to 15.1), sarcoidosis (OR, 14.1; 95% CI, 5.4 to 36.8), and immune thrombocytopenic purpura (OR, ∞; P = 0.022). A statistically significant increase in the risk of Hodgkin’s lymphoma was associated with family histories of sarcoidosis (OR, 1.8; 95% CI, 1.01 to 3.1) and ulcerative colitis (OR, 1.6; 95% CI, 1.02 to 2.6).6 Shared susceptibility for autoimmune disease and lymphoma is suggested by the association between personal and family history for some of these disorders, particularly sarcoidosis, and a statistically significantly increased risk of Hodgkin’s lymphoma, but was not confirmed in a large registry-based study of patients with early rheumatoid arthritis from Sweden.6,7
The occurrence of cancer has a profound effect on the already compromised quality of life of patients with rheumatic diseases and may affect survivorship. A population-based study of cancer survival in patients with inflammatory arthritis from Great Britain suggests decreased survival compared with the general population.8 Survivorship related to the occurrence of cancer in this population was unrelated to disease-modifying antirheumatic drug (DMARD) exposure.8
Rheumatoid Arthritis
A link between lymphoma and rheumatoid arthritis was first reported from a medical record linkage study in 1978.9 Subsequently, a considerable body of evidence emerged supporting rheumatoid arthritis as a pathogenic factor in the development of lymphoma. A standardized incidence ratio (SIR) of 2.4 for lymphoma was described in a population of more than 20,000 Danish patients, as was an increased risk of 1.9 in 1852 U.S. patients.10,11
Using a different approach, a pooled SIR of 3.9 for lymphoma was found using a random effects model in a meta-analysis.12 Studies using other methods have found odds ratios of 1.3 to 1.5 for lymphoma in case-control studies.13
Patients with rheumatoid arthritis may be at higher risk for non-Hodgkin’s lymphoma, especially diffuse large B cell type.14 Large B cell lymphomas represent up to two-thirds of non-Hodgkin’s lymphomas in patients with rheumatoid arthritis6,8—about twice the rate of diffuse large B cell lymphoma as a proportion of overall non-Hodgkin’s lymphoma in the general population. However, other studies have suggested that the immunophenotype, grade, and histology of lymphomas in patients with rheumatoid arthritis are not different from the general population.13
In many studies, the risk of cancer is particularly increased early in the disease course, and cancer risk appears to be greater in patients who have persistently high disease activity, high cumulative disease activity, and more severe disease, and in those who have positive rheumatoid factor (SIR, 3.6; 95% CI, 1.3 to 7.8).15,16 The unadjusted OR for average disease activity comparing highest versus lowest quartile was 71.3 (95% CI, 24.1 to 211.4), and the OR for cumulative disease activity of the 10th decile versus the 1st decile was 61.6 (95% CI, 21.0 to 181.0) in a case-control registry study from Sweden.16
Extra-articular disease of rheumatoid arthritis, particularly Felty’s syndrome and Sjögren’s syndrome, confers a further increased risk of non-Hodgkin’s lymphoma; one study of 906 men with rheumatoid arthritis revealed a twofold increase in total cancer incidence among patients with rheumatoid arthritis who have Felty’s syndrome.17 Large granular T cell leukemia (T-LGL) may rarely occur in association with rheumatoid arthritis.18 T-LGL in rheumatoid arthritis usually is chronic and rarely becomes aggressive.
Whether patients with rheumatoid arthritis are at higher risk for lymphoproliferative disorders other than lymphoma is unclear. At least one study from Canada noted an increased risk of leukemia with an SIR of 2.47 among patients with rheumatoid arthritis. It is interesting to note that an increased risk of lymphoma was not noted in this patient population.19 A U.S. Veterans Affairs study of 906 men with rheumatoid arthritis reported a twofold increased risk of overall cancer, although no single form of cancer stood out, other than non-Hodgkin’s lymphoma, for which a 12-fold increased risk was reported.17
A study that used statewide discharge records from California linking rheumatoid arthritis to Cancer Registry data for 1991 to 2002 revealed 5533 incident cancers among 84,075 rheumatoid arthritis patients observed for approximately 400,000 person-years.20 As in other studies, the risk of developing lymphoproliferative cancers was significantly higher among both women and men with rheumatoid arthritis. Men had significantly higher risks for lung, liver, and esophageal cancers, although a lower risk for prostate cancer was noted. Females were at decreased risk for several cancers, including cancers of the breast, ovary, uterus, cervix, and melanoma, and risk reduction ranged from 15% to 57%, compared with the general population. In this study, Hispanic patients had increased risk of leukemia and vaginal/vulva, lung, and liver cancers.20
The risk of premature death from leukemia and lymphoma in patients with rheumatoid arthritis has been reported to be similar or moderately increased compared with patients without rheumatoid arthritis.21,22 The rate per 100 patient deaths for leukemia/lymphoma is 1.78, including patients who have been treated with methotrexate and azathioprine.22
A meta-analysis of 21 publications from 1990 to 2007 summarized the risk of malignancy in patients with rheumatoid arthritis.23 The risk of lymphoma was increased approximately twofold (SIR, 2.08; 95% CI, 1.8 to 2.39), with greater risks of Hodgkin’s and non-Hodgkin’s lymphoma. The risk of lung cancer was increased with an SIR of 1.63 (95% CI, 1.43 to 1.87). The risk of colorectal cancer was decreased (SIR, 0.77; 95% CI, 0.65 to 0.90), as was the risk of breast cancer (SIR, 0.84; 95% CI, 0.79 to 0.90). The overall SIR for malignancy was slightly increased at 1.05 (95% CI, 1.01 to 1.09). The overall increased risk of cancer in patients with rheumatoid arthritis was largely driven by increased risks of lymphoproliferative cancers.
The risk of lung cancer was also higher among patients with rheumatoid arthritis in the U.S. veteran population. Patients with rheumatoid arthritis were 43% more likely (OR, 1.43) to develop lung cancer compared with patients without rheumatoid arthritis after adjustments for age, gender, race, and tobacco and asbestos exposure.24 No effect of lung cancer on life expectancy was found in a retrospective cohort study; adenocarcinoma was the major histologic form of cancer found in patients with rheumatoid arthritis and in controls. Rheumatoid arthritis appeared to have no influence on the lung cancer stage.25
A study of 42,262 patients hospitalized with rheumatoid arthritis from 1980 to 2004 indexed to the Swedish national cancer registry found that SIRs for Hodgkin’s and non-Hodgkin’s lymphoma were increased for upper digestive tract cancers and for squamous cell skin cancer, and detected an excess of nonthyroid endocrine tumors in patients with rheumatoid arthritis.26 Colon and rectal and endometrial cancers were less frequent in patients with rheumatoid arthritis. The decreased risk of colorectal cancer may be attributable to the use of long-term nonsteroidal anti-inflammatory agents in patients with rheumatoid arthritis.27
In summary, cancer is frequent in the general population and is at least as common among patients with rheumatoid arthritis. Following a diagnosis of rheumatoid arthritis at the typical age of 55 years, one in five patients will be diagnosed with cancer; however, in the great majority of patients, the cancer cannot be linked to rheumatoid arthritis or to its treatment but rather reflects the background cancer risk. Although a history of previous lymphoma does not appear to be a risk factor for developing rheumatoid arthritis, risks of certain cancers, particularly hematopoietic malignancies, are increased and are related to the disease itself.7
Systemic Lupus Erythematosus
Key Points
The risk of lymphoma is at least twofold increased in systemic lupus erythematosus (SLE).
The risk of at least certain malignancies appears to be increased in patients with SLE. Overall SIR ranges from 1.1 to 2.6,28 with the most increased risk being that of lymphomas (SIR of 3.57 for non-Hodgkin’s lymphoma and 2.35 for Hodgkin’s disease).28,29 The risk is especially high for diffuse large B cell lymphoma, often of aggressive subtypes.30,31
A large multicenter (23 centers) international cohort of 9547 patients with an average follow-up of 8 years confirmed an increased risk of cancer in patients with SLE. For all cancers combined, the SIR estimate was 1.15 (95% CI, 1.05 to 1.27); for all hematologic malignancies, it was 2.75 (95% CI, 2.13 to 3.49); and for non-Hodgkin’s lymphoma, it was 3.65 (95% CI, 2.63 to 4.93). The data also suggest increased risks of lung cancer (SIR, 1.37; 95% CI, 1.05 to 1.76) and hepatobiliary cancer (SIR, 2.60; 95% CI, 1.25 to 4.78).29
Patients with SLE in a California statewide patient hospital discharge database from 1991 to 2001 were followed using Cancer Registry data to compare observed versus expected numbers of cancers based on age, sex, and specific incidence rates in the California population.32 A total of 30,478 SLE patients were observed for 157,969 person-years. There were a total of 1,273 cancers for an overall significantly increased cancer risk (SIR, 1.14; 95% CI, 1.07 to 1.20). Patients with SLE had higher risks of vagina/vulva (SIR, 3.27; 95% CI, 2.41 to 4.31) and liver cancers (SIR, 2.70; 95% CI, 1.54 to 4.24). Also, elevated risks of lung, kidney, and thyroid cancers and hematopoietic malignancies were observed with lower rates of screenable cancers, including breast cancer, cervix cancer, and prostate cancer. Drug effects were not assessed.32
Other studies have reported possibly increased risk of malignancies other than lymphoproliferative cancers in SLE. Patients may be at slightly higher risk of thyroid cancer.33 An increased risk of squamous cell skin cancer was found in 238 patients with SLE.34 The risk of breast cancer may be increased by about 1.5-fold to twofold compared with the general population, even after consideration of age, parity, family history, and exogenous estrogens.28,35 The risk of abnormal Pap smears and cervical dysplasia appears to be higher in women with SLE than in those without SLE, although the risk of invasive cervical cancer is not increased.29
The origin of any risk of the development of malignant disorders in SLE remains unclear, although it does not appear to be related to the use of immunosuppressive or cytotoxic agents; most cohorts are too small to allow detection of a statistically meaningful risk increase in rare events over short periods of observation. Race and ethnicity have not been identified as major factors in cancer risk in SLE.36 Antimalarial drug use does not appear to affect the relative risk of malignancy, as was postulated in early studies.37
Risk factors for the development of hematologic malignancies may relate to inflammatory burden and disease activity, immunologic defects and overexpression of Bcl-2 oncogenes, and viruses, especially Epstein-Barr virus (EBV).38 A nested case-control study that included 6438 patients with SLE linked to the national cancer registry in Sweden found that leukopenia, independent of immunosuppressive treatment, was a risk factor for developing these leukemias. Bone marrow investigation was suggested for SLE patients with long-standing leukopenia and anemia.39 Disease characteristics predisposing to non-Hodgkin’s lymphoma include longer disease duration and increased disease activity with moderately severe end-organ damage.40
Women with SLE, likely out of concern for treatment side effects and effects of pregnancy on disease control, are less often exposed to oral contraceptives and are more likely to be nulliparous, which may affect their malignancy risk. On the other hand, the possibly increased breast cancer risk suggests that other, poorly understood factors may increase this risk in women with SLE, whereas at least one study suggested that patients with SLE are less likely than healthy women to undergo breast cancer screening.41 Patients with SLE also appear to be less likely to undergo routine Pap testing. Increased prevalence of human papillomavirus infection and immunosuppression have been implicated in the apparently increased prevalence of abnormal Pap smears and cervical dysplasia in patients with SLE.42
Women with SLE may be at higher risk of lung cancer, for which smoking is a predictor.38 Similar to rheumatoid arthritis, smoking is a risk factor for developing both SLE and lung cancer, reflecting a complex interplay of disease susceptibility factors.
Systemic Sclerosis (Scleroderma)
The risk of malignancy in patients with scleroderma appears to be increased in most reviews and reports, although at least one population-based study failed to detect increased risk.43,44 Generally, estimates of malignancy risk in scleroderma range from SIRs of 1.5 to 5.1, compared with the general population.45,46 The highest SIRs for individual cancers are those for lung cancer, with an incidence ratio of up to 7.8, and non-Hodgkin’s lymphoma, with an incidence rate ratio (IRR) of 9.6.
A population-based disease registry and cancer registry retrospective cohort linkage study from Sweden following patients from 1965 to 1983 revealed an SIR of 1.5 for overall cancer, with highest rates for lung cancer (SIR, 4.9), skin cancer (SIR, 4.2), hepatoma (SIR, 3.3), and hematopoietic malignancies (SIR, 2.3).45 A cohort study of patients followed from 1987 to 2002 revealed a similar magnitude of increased overall risk of malignancy (SIR, 1.55) with the observation of markedly increased risks of oropharyngeal cancer (SIR, 9.63; 95% CI, 2.97 to 16.3) and esophageal cancer (SIR, 15.9; 95% CI, 4.2 to 27.6).45 Esophageal disease related to systemic sclerosis is the likely reason for the increased incidence of Barrett’s esophagus, which has been reported to be present in 12.7% of patients with scleroderma.46
A high rate of abnormal Pap tests in women with onset of scleroderma before the age of 50 has been reported, with a lifetime prevalence by self-report of 25.4% (95% CI, 20.9 to 30.4) compared with a self-reported prevalence of abnormal Pap tests in the general Canadian population of 13.8% (95% CI, 11.6 to 16.4). A significant relationship was found between self-reported abnormal Pap tests and diffuse disease and younger age at disease onset.47
Lung cancer has been reported to account for up to 30% of all cancers in patients with scleroderma; it is thought to be related to fibrosis and, in several studies, not to smoking. The exact nature of the relationship is still unclear. A study of lung cancer occurring in scleroderma patients who were from a population with an already higher than expected rate of lung cancer revealed similar lung cancer rates in both cohorts. Another study from Australia suggested that patients who smoked were seven times more likely to develop lung cancer than those who did not smoke.44 How the incidence rate of cancer in the referent population influences the estimation of rates of cancer in patients with scleroderma was also evident in a registry study from Detroit, in which an increased risk of cancer was not confirmed.44,48 This study included African-American females with scleroderma who appeared to have significantly higher rates of liver cancer (SIR, 45.8).44
The mechanisms of malignancy in scleroderma are largely unexplored. Mechanisms likely vary by individual patient susceptibility and by cancer type and disease-specific factors. Risk factors for development of malignancy in patients with scleroderma may be related to inflammation and fibrosis of affected organs. The link to smoking is controversial.44,48 As in some other autoimmune rheumatic diseases, the risk appears higher early in the disease, and patients who are older at the time of diagnosis may be at higher risk as well.45,49 It is not certain whether the presence of scleroderma-specific antibodies, particularly topoisomerase I (Scl-70), defines an increased risk for development of cancer in these patients.43,49 RNA polymerase I/III autoantibody response in malignancy may initiate the scleroderma-specific immune response and drive disease in a subset of scleroderma patients.50 In contrast to systemic sclerosis, localized scleroderma, including morphea and linear scleroderma, has not been associated with increased risk of cancer.51
Idiopathic Inflammatory Myopathy
Assessment of risk is complicated by the temporal relationship between the development of malignancy and cancer. In particular, some cancers pre-date the onset of inflammatory myopathy, so that the inflammatory myopathy can be better considered a paraneoplastic syndrome (see Chapter 122); it is also likely that the presence of inflammatory myopathies represents a risk factor for the subsequent development of malignancy.52
The incidence of cancer occurring in patients with inflammatory myositis is approximately five to seven times higher than in the general population.53 The prevalence of malignancy is about 25%; cancers are reported more frequently in dermatomyositis, occurring in 6% to 60% of patients, and in 0 to 28% of patients with polymyositis.54
In most studies, cancers manifest within 2 years before or after the initial diagnosis of inflammatory myopathy.55,56 Inflammatory myopathies may initially manifest with the recurrence of a previously diagnosed cancer. A previously diagnosed but inactive inflammatory myopathy may become reactivated with occurrence of a cancer, supporting the hypothesis of autoantigens as drivers of the inflammatory disease.
The strength of the association between malignancy and inflammatory myositis varies. One study done at the Mayo Clinic failed to reveal an increased risk of malignancy in patients with inflammatory myopathy, and a more recent registry study from Sweden of 788 patients diagnosed with dermatomyositis or polymyositis between 1963 and 1987 revealed that 15% of 392 patients with dermatomyositis had cancer diagnosed concurrently with or after the diagnosis of dermatomyositis, with a relative risk of cancer of 2.4 (95% CI, 1.6 to 3.6) for males and 3.4 (95% CI, 2.4 to 4.7) for females.52,55 Of 396 patients with polymyositis, 9% had cancer at or after the time of diagnosis of polymyositis, with the relative risk of 1.8 for development of cancer (95% CI, 1.1 to 2.7) in males and 1.7 (95% CI, 1.0 to 2.5) in females.
A population-based retrospective cohort study from Victoria, Australia, of 537 patients with biopsy-proven dermatomyositis and polymyositis reported a relative risk for malignancy in dermatomyositis compared with polymyositis of 2.4 (95% CI, 1.3 to 4.2) with a higher SIR for dermatomyositis than polymyositis (6.2 vs. 2.0).56 Finally, odds ratios for association of cancer with dermatomyositis from a large meta-analysis were reported to be 4.4, and for polymyositis, 2.1.57
A wide range of malignancies are associated with dermatomyositis and polymyositis. The most common malignancies in populations of Northern European descent are adenocarcinomas of the cervix, lungs, ovaries, pancreas, bladder, and stomach, which account for more than two-thirds of these cancers.58,59 In patients from Southeast Asia, a higher proportion of nasopharyngeal cancers are found, followed by lung cancer.58
The association of cancer is less well understood for more unusual forms of inflammatory myopathies. Amyopathic dermatomyositis, a rare form of dermatomyositis with typical cutaneous but no muscle involvement, can be associated with the development of cancer, but the frequency of this condition is low, so that no stable estimates of cancer risk are available.59 Inclusion body myositis has not been well studied for the same reasons, although the overall risk of cancer of 2.4 suggests a possible link.56
It is likely that relevant antigens are expressed in the underlying tumor and affected muscle. Myositis-specific antigens develop during the process of regeneration in patients who have myositis; these are the same antigens expressed in some cancers known to be associated with the development of inflammatory myopathies.60 A link between malignancy and inflammatory myositis is further supported by observations that in many cases, myositis improves after removal of the malignancy.61
Clinical disease characteristics that may portend higher malignancy risk include active inflammatory disease with normal serum levels of creatine kinase, distal extremity weakness, pharyngeal and diaphragmatic involvement, and leukocytoclastic vasculitis.62–64 Other independent risk factors for the development of cancer in patients who have dermatomyositis in one study of 92 patients included age at diagnosis greater than 52 years (hazard ratio [HR], 7.24; 95% CI, 2.35 to 22.41), rapid onset of skin and/or muscle symptoms (HR, 3.11; 95% CI, 1.07 to 9.02), periungual erythema (HR, 3.93; 95% CI, 1.16 to 13.24), low baseline level of complement factor C4 (HR, 2.74; 95% CI, 1.11 to 6.75), and possibly topoisomerase I.65,66 A low baseline lymphocyte count was a protective factor for malignancy (HR, 0.33; 95% CI, 0.14 to 0.80), although the number of assessable patients was small.65
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree