CHAPTER 1 Biology of Healing and Tissue Repair
ROTATOR CUFF PATHOLOGY
Natural History of Rotator Cuff Tears
In the diagnosis and treatment of rotator cuff pathology, it is important to understand when tears occur and how they progress. Using ultrasound and magnetic resonance imaging (MRI), there is strong evidence to show that the incidence of rotator cuff tears increases with age. When ultrasound was used, asymptomatic subjects demonstrated tear prevalence of 13% in their sixth decade, which increased to 51% in their ninth decade; there was an overall tear prevalence of 23% in subjects older than 50 years.1 Using MRI as an imaging tool, the prevalence of asymptomatic tears was found to be 4% in subjects younger than 40 years, dramatically increasing to 40% in subjects older than 50 years.2
Yamaguchi and colleagues3 have investigated not only the prevalence of rotator cuff tears, but also how these tears progress. They used ultrasound to screen patients presenting with unilateral shoulder pain and found that 40% of these patients had full-thickness tears in their contralateral asymptomatic shoulder, of which 51% became symptomatic within 3 years and none decreased in size. They observed a risk for tear and symptom progression with time. In a further study, they found a strong correlation between the prevalence of rotator cuff tears and age, while demonstrating that larger tears were more symptomatic.4 The symptomatic tears were 30% larger than asymptomatic tears. Based on these data, this group has recommended routine ultrasound follow-up for patients treated nonoperatively.
Rotator Cuff Degeneration
Intrinsic Factors
There is histologic and molecular evidence of intrinsic tendon degenerative changes associated with tendinopathy. Evaluation of tissue taken from torn rotator cuffs at the time of repair demonstrates histologic degenerative changes. Hashimoto and associates5 have described characteristics of age-related degeneration, including thinned and disoriented collagen fibers with chondroid metaplasia, myxoid and hyaline degeneration, calcification, fatty infiltration, and vascular proliferation. Biochemical alterations in the rotator cuff tendon have also been demonstrated with aging. In the aged tendon, the more mechanically resistant type II collagen is replaced with type III collagen, and there is increased tendon calcification and microtearing at the tendon-bone insertion.6 Additionally, elevated levels of alpha–smooth muscle actin (SMA) have been found at the edges of torn human rotator cuff tendons.7 SMA has been postulated to be involved in tendon retraction and is stimulated by the cytokine transforming growth factor beta 1 (TGF-β1).
Microtrauma is another hypothesized cause of intrinsic degeneration. It is theorized that repetitive tendon overload leads to small cuff injuries and, ultimately, full-thickness tears. This theory is consistent with increased prevalence of articular-sided supraspinatus tears, because higher tensions have been shown to occur on the articular half of this tendon. Soslowsky and coworkers8 have proposed a rat model for this repetitive degeneration. They found that rats exposed to repetitive exercise demonstrate signs of rotator cuff tendinopathy similar to those seen in human degenerative tendons.
Extrinsic Factors
Extrinsic factors contributing to rotator cuff pathology include anatomic, environmental, and demographic factors. Neer and Poppen8A first introduced the theory of mechanical impingement on the rotator cuff. Based on intraoperative observations, they hypothesized that bursal-sided lesions were likely caused by the anterior acromion. Correspondingly, Bigliani8B defined acromial morphologies that were more associated with rotator cuff tears. These acromial “spurs” were initially thought to be congenital, but studies have demonstrated an age-acquired progression that may be traction-related.9
In rotator cuff tendinopathy, acromial impingement does not act in isolation, because it has been shown that acromioplasty does not prevent later rotator cuff tears.10 However, Ko and colleagues have provided evidence that bursal-sided tears are likely caused by impingement, whereas articular-sided tears are derived from other degenerative processes.11 They recommended performing acromioplasty with bursal-sided tearing, but only if there is obvious spurring with isolated articular-sided tears.
Impingement that is attributed to functional or static instability has been termed secondary impingement. This imbalance can be to the result of neurologic factors, capsular laxity or contracture, or a combination of factors. Other extrinsic factors include overuse; some have found an increased incidence of tendinopathy in dominant extremities.4
Vascularity
Vascular changes have long been postulated to play an important role in degeneration, but this remains controversial. Historically, several groups have looked at the histology in the critical zone medial to the tendon-bone attachment to evaluate the vascularity and its potential role in tendinopathy. In a more recent study, Brooks and associates12 evaluated this area and found decreased vascularity within 15 mm of the rotator cuff insertion in both the supraspinatus and infraspinatus. They concluded that other factors are responsible for tendinopathy, because they believed that tendinopathy is more prevalent in the supraspinatus than the infraspinatus. However, they did not suggest the possibility that vascularity may be part of a multifactorial process.
More recently, Rudzki and coworkers13 used contrast-enhanced ultrasound to show a statistically significant decrease in supraspinatus vascularity at the critical zone in asymptomatic subjects older than 40 years with intact rotator cuffs. This was consistently most pronounced at the articular surface of the tendon as it approaches the medial footprint. Biberthaler and colleagues,14 in another human in vivo model, used polarized imaging to measure functional capillary density at the time of arthroscopy and found quantitatively decreased vascularity at the edge of degenerative rotator cuff lesions. These results suggest the possibility of a vascular contribution to the pathogenesis of rotator cuff tendinopathy that occurs with aging.
Neuropeptides
The possibility of neuropeptides contributing to tendinopathy has recently been introduced. Glutamate, substance P, and serotonin are neuropeptides that have been investigated in tendinopathy. Increased expression of glutamate signaling proteins has been demonstrated in rat supraspinatus tendons subjected to overuse. Additionally, tenocytes exposed to glutamate have increased rates of apoptosis.15 Substance P has been shown to be elevated in the bursa of diseased rotator cuff tendons.16
Tendinopathy as a Multifactorial Process
Rotator cuff tendinopathy is likely caused by multiple factors and it would be shortsighted to imply that there is only one cause. Soslowsky and associates17 have investigated the overlap of intrinsic and extrinsic factors by using the aforementioned rat tendon overuse model, along with a model of extrinsic compression. This study demonstrated that tendon injury created through the combination of their microtrauma model and their extrinsic compression model was significantly greater than either in isolation.
Apoptosis
Apoptosis has been shown to be associated with rotator cuff disease. Yuan and coworkers18 have found that apoptotic cell proportion is increased from 13% in controls to 34% at the edge of rotator cuff tears in humans. They have also shown increased tenocyte production of apoptotic mediators in response to in vitro oxidative stress. Additionally, they found an upregulation of the antioxidant peroxiredoxin 5 (PRDX5) in degenerative rotator cuff tendons. Interestingly, PRDX5 in vitro has been shown to decrease apoptosis and increase collagen synthesis. Heat shock proteins are other cell-protective molecules associated with apoptosis in rotator cuff tendinopathy.19
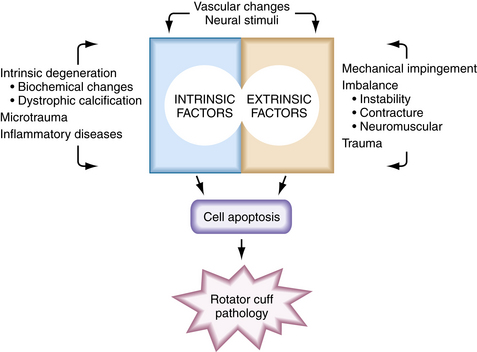
The mechanism whereby oxidative and other stress factors induce tenocyte apoptosis has not been fully established. However, the protein-activating enzyme c-Jun N-terminal protein kinase (JNK) has been implicated. JNK has been shown to activate transcription factors in the apoptotic pathway and to be linked with matrix metalloprotease-1 (MMP-1) in torn human supraspinatus tendons.20 Data suggest that stress-induced JNK may activate MMP-1, which in turn may be responsible for apoptotic tissue degeneration. These molecules may become potential therapeutic targets. Figure 1-1 summarizes the degenerative factors discussed in this section.
BIOLOGIC PROCESS OF ROTATOR CUFF HEALING
Anatomy of the Tendon-Bone Insertion and Rotator Cuff Healing
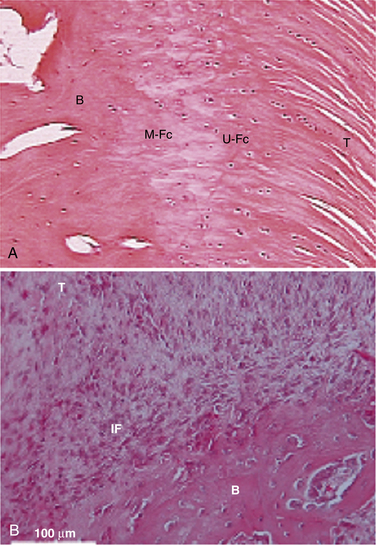
Traditionally, we have evaluated rotator cuff healing by investigating tendon-bone healing. The normal anatomy of the tendon-bone interface consists of a four-zone transition, including tendon, fibrocartilage, calcified fibrocartilage, and bone.21,22 The histiologic reconstitution of this bone-tendon interface in rotator cuff repairs has been shown to occur by reactive scar formation, which fails to recreate the native interface (Fig. 1-2). Preservation of the native transitional zone is clinically important to mitigate changes in mechanical properties between tendon and bone and to protect our repairs, because the mechanical properties of fibrous scar are less robust.23,24 Thus, creating a healing environment for scarless healing may be the next biologic hurdle to improve repair strength and decrease failure rate.
It has been postulated that the influx of macrophages and subsequent cytokine production leads to formation of the fibrous scar tissue at the tendon-bone insertion site. This hypothesis is derived from the study of fetal wounds in which tissue regeneration occurs, rather than scar formation. Through the study of knockout mice, it has been shown that healing rates are not affected by the absence of macrophages and neutrophils, suggesting that the initial inflammatory response may not be necessary to healing.25 Hays and colleagues26 used an anterior cruciate ligament (ACL) reconstruction model in macrophage-depleted rats and showed improved biomechanical and morphologic healing properties at the tendon-bone interface, likely as a result of the lack of cytokine-producing macrophages.
Origins of the Rotator Cuff Healing Response
Uhthoff and associates27 have studied which tissues are responsible for rotator cuff healing in a rabbit model 2 weeks after an experimental rotator cuff repair. They found no cellular or vascular healing at the tendon stump, but did find increased cellularity and vascularity in the cancellous bone and bursa. They concluded that it is likely better to avoid significant débridement of the tendon stump for reparative tissue volume and to attempt to preserve subacromial bursa.
Hirose and coworkers28 have researched rotator cuff healing in rabbits and found that reparative cells are derived from the bursal-sided epitenon, without evidence of the continuity of reparative tissue to the subacromial bursa. This is consistent with clinical data that showed comparable results in arthroscopic rotator cuff repair with bursectomy and in open surgery in which the bursa is spared.29 Lo and associates30 found upregulation of collagen and proteoglycans involved with healing at the bursa and rotator cuff tear margins in patients undergoing rotator cuff repairs and concluded that both the tendon and bursa contribute to healing.
Biologic Factors in Rotator Cuff Healing
Inflammatory Response.
The inflammatory response is the first phase of rotator cuff healing. Within a few days of cuff repair, proinflammatory neutrophils and recruited phagocytic macrophages are seen at the tendon-bone interface. Within 7 to 10 days, resident macrophages and lymphocytes are present in a proregenerative response.21,31 Cytokines are the mediators of early healing and are released and stimulated by these cells.
Cytokines.
Cytokines are molecules that modulate and influence cell recruitment, proliferation, differentiation, and matrix synthesis. Cytokines involved in bone and tendon healing include TGF-β, interleukins (ILs), bone morphogenic proteins (BMPs), insulin-like growth factors (IGFs), vascular endothelial growth factors (VEGFs), and platelet-derived growth factors (PDGFs). The interplay of these factors in tendon healing is complicated. Their expression in rotator cuff healing seems to peak from about 1 to 2 weeks, and can be upregulated for weeks before returning to baseline by 16 weeks.21
Cytokines have been shown to be an integral part of rotator cuff healing; transgenic knockout mice studies have demonstrated that IL-4 and IL-6 are required modulators of the healing response.32 The cytokine PDGF-β has also been shown to augment healing in a rat rotator cuff repair model through restoration of the normal crimp pattern and collagen bundle alignment that were not seen in nonaugmented repairs.33
Bone Morphogenic Proteins
Special categories of cytokines are tissue inductive molecules, such as BMPs, now ubiquitous in the orthopedic literature. These molecules, particularly BMPs 2 through 7, have been shown to be osteoinductive. Because bone-tendon healing depends on bone ingrowth, it is reasonable that the addition of BMP may affect healing. Rodeo and colleagues34 looked at this possibility, in a sheep model, by supplementing rotator cuff repairs with BMP-2 through BMP-7, along with other cytokines. They demonstrated increased reparative tissue in the augmented repairs with increased failure loads. However, when the reparative tissue was normalized to tissue volume, failure loads were identical, which implies that the supplemental healing was quantitative and not qualitative.
Other BMPs, such as BMP-12 and BMP-14 molecules, are more specific to tendon insertion sites, and induce embryologic tendon and fibrocartilage formation. Using a sheep model, with rotator cuff repairs augmented with recombinant human BMP-12 in a collagen or hyaluronan sponge, repairs were two to three times stronger in load to failure and stiffness at 8 weeks. Histologic examination yielded more advanced healing in the BMP-12 group, although it was still qualitatively inferior to the native tendon-bone interface. These data suggest that BMPs may have the potential to supplement repairs through accelerated healing and rehabilitation.35 In knockout mice studies, BMP-14 has been shown to be essential in the modulation of tendon healing.36 Additionally, in human full-thickness rotator cuff tears, BMP-14 mRNA was found in high concentrations at the bursal side of the torn edge of the tendon in a similar regional distribution as type I collagen, implying a roll in the reparative process.37
Matrix Metalloproteases.
MMPs are catabolic enzymes that appear to be involved in apoptosis and the remodeling phase of rotator cuff healing. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous molecules know to inhibit MMPs. Choi and associates38 have explored the spontaneous healing of full-thickness rotator cuff tears in rabbits. Their data demonstrated MMP-2 expression and activation at both edges of the torn tendon during healing, with reparative tissue encroaching from the bursal side. TIMP-1 and -2 were also expressed at the cut tendon edges, as well as in the reparative tissue.
Collagen and Extracellular Matrix Molecules.
Collagen and matrix proteins are the structural components of tendon healing. Tendons consist primarily of collagen, specifically tendons, which are 95% type I collagen. Other collagen in tendons include types II, V, and VI. Proteoglycans, negatively charged macromolecules, and other matrix molecules make up the other components of tendons that contribute their unique mechanical properties. These molecules are regulated during the initial week to months of healing and are involved in rotator cuff healing and remodeling.21,39
In both rat and human rotator cuff tendon repair studies, increased collagen expression has been demonstrated; specifically, type I collagen was elevated in both species. Additionally, specific proteoglycans were suppressed and stimulated. Decorin was decreased, whereas aggrecan was increased across both species.30,39 These data demonstrate that regulation of extracellular matrix molecules is an integral part of rotator cuff healing.
Vascularity in Rotator Cuff Healing.
The role of vascularity is unclear in rotator cuff healing, not unlike the role of vascularity in tendinopathy. Capillary proliferation has been shown to occur in rat rotator cuff repair healing at 3 days and peak at 10 days.21 Fealy and coworkers40 have investigated vascularity after rotator cuff repair clinically using sonography. They found a robust vascular response in the repaired tendon, which predictably decreased over 6 months. The response was most prevalent at the peritendinous region and lowest at the bony attachment site. Vascular scores did not differ in repairs with or without defects.