4
Basic Principles of Pharmacology
Dorraine Reynolds and Micki Cuppett
At the completion of this chapter the reader should be able to do the following:
1. Describe the regulation of pharmaceuticals, including the management of prescription and nonprescription medication in an athletic training facility
2. Describe the basics of pharmaceutics and pharmacokinetics including dosage forms, routes of administration, and drug storage
3. Access various drug information resources to obtain more information about a drug
Introduction
This chapter introduces the reader to the regulatory, pharmacological, and pharmaceutical information that athletic trainers or other allied health professionals must be familiar with and understand. Important regulatory agencies discussed include the U.S. Food and Drug Administration (FDA), the U.S. Drug Enforcement Administration (DEA), and the National Collegiate Athletic Association (NCAA). The basic principles of pharmacology cover pharmacokinetics, routes of administration, drug storage and resources, pharmacodynamics, indications and contraindications, and special considerations for athletes. Drug information resources also are reviewed and include web-based and mobile options. This chapter is designed as an overview and lists additional sources for continued reading and expanded content. Information about specific drug classifications is found in Chapter 5.
Regulation of Pharmaceuticals
It is important for athletic trainers to understand the legal regulations that apply to the use of medications. Two entities of the U.S. federal government are charged to control the use of pharmaceutical products: the Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA). The agency in Canada equivalent to the FDA is the Canadian Health Products and Food Branch (HPFB). The HPFB is responsible for regulating food and health products, including pharmaceuticals, biological agents, and genetic therapies.1 The HPFB is one of nine branches of Health Canada, which all report to the deputy and associate deputy ministers of health.
Drug Enforcement Administration
Controlled substances are drugs that have a high potential for abuse (e.g., morphine and codeine). The DEA has established five categories or “schedules” of controlled substances (I-V). Table 4-1 lists drugs that are classified as controlled substances and are placed into one of these five categories on the basis of their likelihood for abuse.
TABLE 4-1
Drug Enforcement Administration Schedules of Controlled Substances
| Schedule | Description | Examples of Drugs∗ |
| I | ||
| II | ||
| III | Drugs with less abuse potential than those in schedule I or II | |
| IV | Drugs with less abuse potential than those in schedule II | |
| V | <200 mg of codeine or opium per 100 g |
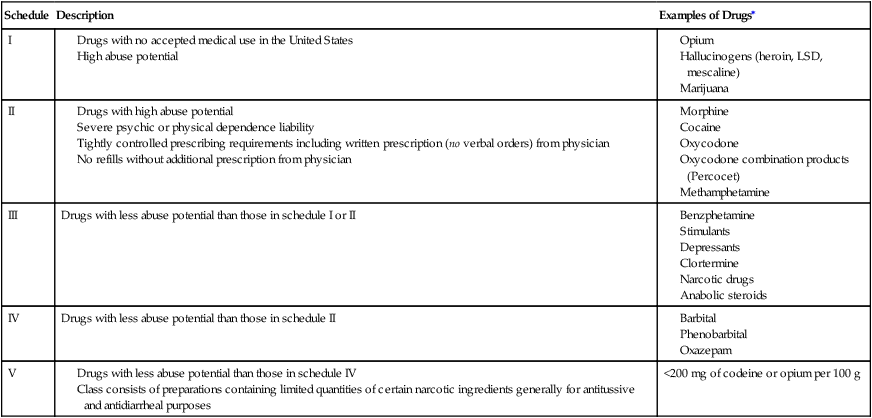
LSD, Lysergic acid diethylamide.
∗Examples of drugs in each schedule do not represent an all-inclusive list.
Food and Drug Administration
The FDA is an agency of the U.S. Department of Health and Human Services. Its mission is to “promote and protect the public health by helping safe and effective products reach the market in a timely way and monitoring products for continued safety after they are in use.”2
how the drug is manufactured. Pharmaceutical product manufacturers must meet stringent manufacturing guidelines and pass FDA inspections. The drug products that they produce must consistently pass dissolution and bioequivalence tests. It is important to remember that the FDA does not oversee the marketing or sale of food supplements and herbal products, many of which are used by athletes, and neither does any other government agency.
Administration versus Dispensing
States have regulations that address both the administration and dispensing of drugs. It is important that the athletic trainer understand the differences between these two actions. Dispensing is the act of delivering a medication to an ultimate user pursuant to a medical order issued by a practitioner authorized to prescribe. This includes the packaging, labeling, or compounding necessary to prepare the medication for such delivery. Administration is the act of applying a medication by injection, inhalation, ingestion, or any other means to the body of a patient in a single dose.3
Both dispensing and administration require a written prescription from a physician. In some states, dispensing medications is beyond the scope of the athletic training profession. It is important that the athletic training facility comply with both state and federal regulations governing prescription medications.4 Some states require that an athletic training facility have a signed agreement with a physician allowing the athletic training staff to serve as agents of the physician in the care of the physician’s patients. At no time does the agent make any discretionary decisions about the administration or dosage of a medication.3 Prescription medications that may be used for on-the-field treatments must be secured and accessed only by the physician.4 Companies such as SportPharm, Inc. are used by several major colleges and universities as well as by professional athletic teams to help ensure compliance with state and federal regulations. The 50 states have different legislation about administration and dispensing of medications. The athletic trainer, team physician, and team pharmacist must work together to ensure that state and federal regulations are being met.
Pharmacology
Routes of Administration
Drugs can enter the body through a variety of routes (Box 4-1), and these paths of administration promote the drug’s absorption. The oral route is certainly the most common for the administration of drugs, but often it is appropriate to use a parenteral (nonoral) route of administration. The preferred route is determined by multiple factors including ease of administration, patient adherence, desired onset of action, local versus systemic distribution, and properties of the drug itself. An example is the destruction of insulin in the gastrointestinal tract, which necessitates its administration through a nonoral route.
Inhaled
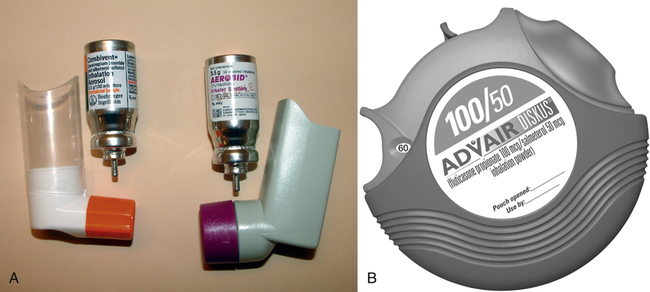
Inhaled drugs are most frequently used for their local effect on the bronchial passages. Bronchodilators and corticosteroids to treat bronchoconstriction resulting from asthma are the drugs most frequently given via this route. These drugs may be administered via metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. MDIs (Figure 4-1, A) and dry powder inhalers (Figure 4-1, B) deliver a set dose of the drug with each inhalation. Nebulizers are machines that use compressed air to cause aerosolization of a liquid drug, which is then inhaled through a mask or a mouthpiece (see Figure 5-6). Intranasal sprays and inhalers are used for a local effect on the intranasal passages. Corticosteroids are the most common drugs delivered by the intranasal route to treat allergic rhinitis.
Ophthalmic
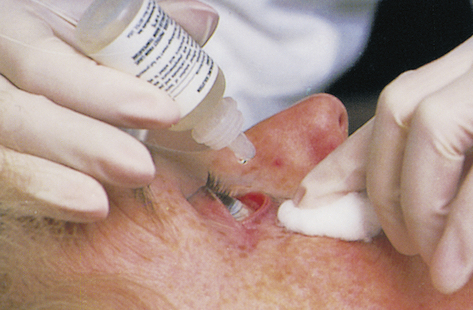
Ophthalmic administration of drugs may be used to treat eye infections, allergies, dryness, glaucoma, and other eye disorders. The droppers used to administer drugs into the eye must be kept sterile, and therefore the eyedropper must never touch the eye. Individuals administering the drops must wash their hands thoroughly before and after administration of the drops. Warming the drops to body temperature by rolling the dropper bottle rapidly between the hands may increase the comfort of ophthalmic administration. Administration of the drops into the lateral area of the eye also increases comfort because the medial area, pupil, and iris are much more sensitive. Applying the drops laterally also helps bathe the eye because tears are produced laterally and flow medially to the nasolacrimal duct (Figure 4-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree