75 Ankylosing Spondylitis
Inflammatory back pain is the usual clue to the early diagnosis of AS and axial spondyloarthritis.
Group physiotherapy is more effective than exercises performed at home by the patient.
Ankylosing spondylitis (AS) belongs to the group of diseases known as the spondyloarthropathies or, better, spondyloarthritides. This group of disorders constitutes a family of related but heterogeneous conditions rather than a single disease with different clinical manifestations1 (Tables 75-1 and 75-2).
Table 75-2 Clinical Characteristics of Spondyloarthritis
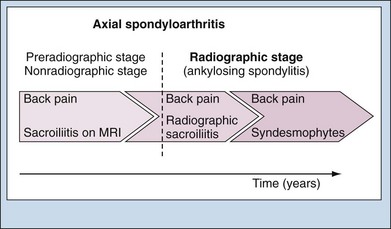
Many patients with AS have onset of back pain during their third decade of life. It takes, on average, 6 to 8 years between the onset of back pain and establishing a definite diagnosis of AS. This diagnostic delay in the majority of patients results mainly from the relatively late appearance of definite radiographic sacroiliitis on conventional plain radiographs,2,3 which is a requirement for diagnosis according to the modified New York criteria. Active sacroiliitis on magnetic resonance imaging (MRI) has been shown to predict the later appearance of sacroiliitis on radiographs.4,5 Thus many patients at an early stage of AS typically present with characteristic clinical symptoms of AS but may not show definite sacroiliitis on radiographs. Therefore they may not be classified as AS cases. In such patients with suspected early AS, sacroiliitis may best be detected on MRI.3 It has been proposed that a substantial proportion of such patients will develop radiographic sacroiliitis (i.e., structural damage of SI joints) with time and will progress to definite AS, but this hypothesis requires further evaluation in prospective studies. On the other hand, this also implies that a proportion of patients will remain at this (nonradiographic) stage of disease, with inflammation on MRI at some point, but without radiographically detectable damage over the subsequent years.5 Therefore in order to describe these patients correctly, the term early AS has been dropped, with terms such as preradiographic axial spondyloarthritis3 or, more recently, nonradiographic axial spondyloarthritis6 seeming to be more appropriate (Figure 75-1). The term axial spondyloarthritis comprises both nonradiographic AS and classic AS (according to the modified New York criteria) and is now considered by ASAS (Assessment of SpondyloArthritis international Society) as the preferred terminology for patients with predominantly spinal disease. The total prevalence of axial spondyloarthritis has been estimated at two to three times that of AS according to the modified New York criteria.7 In the concept of axial spondyloarthritis, the disease AS represents the tip of the iceberg. Patients with axial spondyloarthritis who have normal SI joints on conventional radiographs often show inflammatory changes on appropriate MRI and may or may not progress to sacroiliitis according to the New York criteria. Such patients may have active disease that needs appropriate treatment. They respond to therapy with biologics. The course and natural history of axial spondyloarthritis without radiographic sacroiliitis is not yet well known. However, although this chapter focuses on radiographic AS, many aspects also apply to patients with axial spondyloarthritis. The interval between the first complaints of the disease and the time of a definite diagnosis may be as long as 4 to 9 years.8 This affects how disease duration is defined. Important components of the definition of disease duration are provided in Table 75-3.9
Table 75-3 Components of Disease Duration
AS, ankylosing spondylitis.
From Davis, JC, Dougados M, Braun J, et al: Definition of disease duration in ankylosing spondylitis: reassessing the concept, Ann Rheum Dis 65:1518–1520, 2006.
Classification
Criteria for Ankylosing Spondylitis and Axial Spondyloarthritis
The diagnosis of AS is based on clinical features. The disease is “primary” or “idiopathic” if no associated disorder is present; it is “secondary” if the disease is associated with psoriasis or chronic inflammatory bowel disease. In daily practice, a presumptive clinical diagnosis of AS is usually supported by evidence of sacroiliitis on conventional pelvic radiographs; indeed, many think of AS as symptomatic sacroiliitis. The presence of sacroiliitis does not necessarily indicate the presence of AS, however. Moreover, although radiographic sacroiliitis is frequent in AS, it is by no means an early or obligate manifestation of the disease.10 Contrary to the New York criteria, radiographic evidence of sacroiliitis is not obligatory to fulfill the Rome criteria (Table 75-4). Both sets were primarily intended for use in epidemiologic studies. Lack of either sensitivity or specificity led to a modification of the New York criteria for AS11 (see Table 75-4). Two criteria—limitation of lumbar spine motion and limitation of chest expansion—appear to reflect disease duration; they are usually not present in early disease.12 It should be stressed that classification criteria are usually not useful for early diagnosis owing to a lack of sensitivity. In particular, in the early phase of AS, conventional SI radiographs may be normal. MRI is useful to diagnose the disease with predominantly axial manifestations before the presence of radiographic sacroiliitis.13 To encompass both preradiographic AS and classic AS (according to the modified New York criteria), the Assessment of SpondyloArthritis international Society (ASAS) has proposed classification criteria for axial spondyloarthritis for patients with back pain of at least 3 months’ duration and age at onset of complaints before 45 years (Table 75-5). The term axial spondyloarthritis has been introduced as an umbrella for the entire spectrum of spondyloarthritis patients with predominant axial involvement, irrespective of the presence of structural damage on radiographs. Thus axial spondyloarthritis includes nonradiographic axial spondyloarthritis and classical AS (fulfilling the modified New York criteria). According to the ASAS classification criteria for axial spondyloarthritis,7 a patient with chronic back pain and age at onset before age 45 can be classified as having axial spondyloarthritis if sacroiliitis on imaging (radiographs or MRI) is present plus at least one further spondyloarthritic feature, or, in the absence of sacroiliitis on imaging, if HLA-B27 plus at least two further spondyloarthritis features are present (see Table 75-5). The sensitivity of the entire set of ASAS criteria for axial spondyloarthritis was 83%, and the specificity was 84%.7 In the ASAS study on these new criteria, 30% of all patients diagnosed as having axial spondyloarthritis had definite radiographic sacroiliitis and fulfilled the modified New York criteria for AS. Therefore two-thirds were classified as nonradiographic axial spondyloarthritis.7
Table 75-4 Criteria for Ankylosing Spondylitis
| Rome, 1961 |
| Clinical Criteria |
| Radiographic Criterion |
| Definite Ankylosing Spondylitis |
| New York, 1966 |
| Diagnostic Criteria |
| Grading of Radiographs |
| Definite Ankylosing Spondylitis |
| Probable Ankylosing Spondylitis |
| Modified New York, 1984 |
| Criteria |
| Definite Ankylosing Spondylitis |
Data from van der Linden SM, Valkenburg HA, Cats A: Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria, Arthritis Rheum 27:361–368, 1984.
Table 75-5 ASAS Classification Criteria for Axial Spondyloarthritis (SpA) (in Patients with Back Pain ≥ 3 Months and Age at Onset < 45 Years)
| Sacroiliitis on imaging | OR | HLA-B27 |
| plus | plus | |
| ≥1 SpA feature | ≥2 other SpA features |
| SpA Features | Sacroiliitis on Imaging |
|---|---|
ASAS, Assessment of SpondyloArthritis international Society; CRP, C-reactive protein; HLA-B27, human leukocyte antigen-B27; IBP, inflammatory back pain; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs.
* Elevated CRP is considered a SpA feature in the context of chronic back pain.
From Kollinger S, Bird LA, Roddis M, et al: HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors, J Immunol 173:1699–1710, 2004; and Rudwaleit M: New classification criteria for spondyloarthritis, Int J Adv Rheumatol 8:1–7, 2010.
Epidemiology
Prevalence
The prevalence of AS closely parallels the frequency of HLA-B27. This holds true for those B27 subtypes that are associated with the disease, but it is not true for populations in which the HLA-B27*06 subtype that lacks a strong association with AS occurs frequently such as the Indonesian population.14–16
Among whites, the estimated prevalence rate of AS as defined by the modified New York criteria ranges from 68 per 100,000 population older than 20 years in the Netherlands to 197 per 100,000 in the United States.8,17 The prevalence of clinical AS in France is 150 per 100,000 adults, whereas in Norway it is 210 per 100,000 adults.18,19 The prevalence of the disease in Finland is similar, with a figure of 150 per 100,000 people.20
Higher prevalence rates have been reported in central Europe. An epidemiologic study from Berlin reported a prevalence figure of 0.86%.21 In the general population, AS is likely to develop in about 1% to 2% of HLA-B27+ adults who have a disease-associated B27 subtype, although there may be regional or geographic differences. For example, in northern Norway, AS may develop in 6.7% of HLA-B27+ people.8,22
The disease is much more common among HLA-B27+ first-degree relatives of HLA-B27+ AS patients; roughly 10% to 30% of them have signs or symptoms of AS.8 In fact, a positive family history of AS is a strong risk factor for the disease.
Incidence
There is no adequate evidence that the incidence of AS has changed in the past few decades. Clinical features, age of onset, and survival time have remained stable.23 One study revealed an overall age and gender-adjusted incidence of 7.3 per 100,000 person-years. This U.S. figure compares quite well with the Finnish study, which revealed a stable incidence of 8.7 (95% confidence interval [CI], 6.4 to 11.0) per 100,000 people aged 16 or older.18
Burden of Disease
AS is associated with a considerable burden to the patient and society. Apart from the axial and articular manifestations, extra-articular manifestations such as enthesitis and acute anterior uveitis and comorbidities such as inflammatory bowel disease and psoriasis contribute to the burden of disease. In addition, a large proportion of patients has spinal osteoporosis, leading to vertebral fractures and thoracic kyphosis. All these features result in a decreased quality of life. Disease status scores for physical functioning and disease activity correlate clearly with psychologic scores for anxiety and depression.24 The impact of AS can also be seen in various aspects of employment, ranging from requiring assistance at work to increased sick leave and withdrawal from the workforce.25,26 Apart from the impact on labor force participation, AS patients have an important impact on health care and non–health care resource utilization, resulting in mean total costs (direct and productivity) of about $6700 to $9500 per year per patient when applying the human capital approach to calculate productivity costs.27–29
The burden of illness increases with duration of disease. Because the burden due to AS reduces quality of life, and because all types of costs associated with AS result from loss of function and disease activity, early diagnosis and treatment are necessary to prevent or reduce functional decline and improve patient outcome.30
Pathogenesis
HLA-B27
The dominant role of genetic factors is highlighted by data demonstrating disease concordance in 75% of monozygotic twins compared with 13% of nonidentical twins,31 familial aggregation,32 and population data demonstrating associations with HLA-B27.33 About 90% of white AS patients are HLA-B27+. HLA-B27 is the first described and major genetic risk factor for AS but, despite the near-pervasive nature of this association, it has been estimated that B27 contributes only 16% of the total genetic risk. In humans, there appears to be a hierarchy of association of the more than 45 as yet known subtypes of HLA-B27 with AS, ranging from strong association with HLA-B*2705 to weak association with HLA-B*2709. The direct pathogenic role of HLA-B27 is evidenced by the spontaneous spondyloarthritis-like disease in rats overexpressing HLA-B27.34–36
The main function of human leukocyte antigen (HLA) class I molecules such as HLA-B27 is to present peptides to CD8+ T cells and, accordingly, the arthritogenic peptide hypothesis proposes that CD8+ T cells are activated by bacterial antigens, for example in the gut, and after recirculation are reactivated in the joint by cartilage or other autoantigens. A number of studies have provided some partial evidence for this concept.37,38 This hypothesis has been thoroughly questioned by the fact that HLA-B27 transgenic rats develop severe spondyloarthritis-like disease even in the absence of CD8+ T cells.39,40 Two alternative hypotheses have been proposed more recently, suggesting that HLA-B27 has other specific features independent of antigen presentation that may directly contribute to disease. One theory indicates that HLA-B27 has a special tendency to form heavy chain homodimers that, when present on the cell surface, can trigger direct activation of natural killer (NK) cells through recognition via killer immunoglobulin receptor (KIR)-like receptors.41,42
Alternatively, HLA-B27 has a special propensity to misfold in the endoplasmic reticulum and thereby to promote an unfolded protein response, which in turn modulates the functional behavior and cytokine production of myeloid cells.43–45
Population and family studies have shown that HLA-B60 increases susceptibility to AS.46 This applies to both HLA-B27+ and HLA-B27− persons.
Non–Human Leukocyte Antigen Genes
Two observations clearly suggest a role for non-HLA genetic risk factors. First, there is an increased risk for disease in B27+ first-degree relatives of AS probands (10% to 20%) compared with B27+ individuals in the general population (2% to 5%).8 Second, disease concordance is 75% in identical twins versus 27% in HLA-B27 concordant dizygotic twins.31 Genome-wide association studies have now allowed the identification of several of these factors.47,48 There is a robust association with single-nucleotide polymorphisms (SNPs) in endoplasmic reticulum aminopeptidase-1 (ERAP-1), an enzyme involved in the trimming of peptides for loading in MHC molecules. There is now emerging evidence for a gene-gene interaction between ERAP-1 and HLA-B27, but it remains unknown how the polymorphisms may affect antigen presentation, HLA-B27 homodimer formation, and misfolding. A second strong association is found with SNPs in IL-23R, a genetic feature shared with Crohn’s disease and psoriasis. Together with the suggested but not yet confirmed association with STAT349 and JAK2 polymorphisms, this suggests the potential involvement of an interleukin (IL)-17 response in AS. Definite association was also found with gene deserts on chromosome 2p15 and 21q22, which warrants further investigation of noncoding ribonucleicacid (RNA) and epigenetic effects in the pathophysiology of this disease. Besides these definite associations, genome-wide association studies have suggested potential associations with tumor necrosis factor (TNF) receptor 1 (TNFR1), the signaling molecule TNFR1-associated death domain protein (TRADD), the TNF superfamily cytokine TNFSF15, IL-1A and the IL-1 receptor 2 (IL-1R2), IL-12B, the vascular morphogenesis protein gene anthrax toxin receptor 2 (ANTXR2), and the innate immune receptor caspase recruitment domain family, member 9 (CARD9).50
Autoimmunity versus Autoinflammation
Both the alternative roles for HLA-B27 besides antigen presentation and the genetic risk factors suggest that AS may not be primarily driven by a canonical autoantigen-specific T and/or B cell reactivity. Indeed, AS does not share genetic risk factors such as PTPN22 or cytotoxic T lymphocyte–associated antigen-4 (CTLA-4) with other autoimmune diseases and lacks disease-specific autoantibodies. On the basis of the predilection of the disease for sites rich in cartilage, it has been proposed that cartilage antigens may be the primary target of an autoimmune response in AS. This hypothesis is supported by several animal models of peripheral and axial arthritis on the basis of the induction of autoimmunity to antigens present in cartilage and fibrous tissue such as aggrecan and versican.51,52 T cell responses toward the G1 domain of aggrecan have also been observed in human AS, but similar responses were seen in other inflammatory joint disorders, implying a nonspecific response to joint damage.53 Finally, biologic therapies targeting B (such as anti-CD20) or T (such as CTLA4-Ig) cell pathways have limited to no efficacy in AS in proof-of-concept trials.54,55 Taken together, little strong evidence supports a primary T cell– or B cell–driven autoimmune process in AS.
An alternative hypothesis proposes that AS would be driven by abnormal reactivity of innate immune cells such as macrophages, neutrophils, and mast cells. This hypothesis is supported by several lines of evidence. First, genetic associations with CARD9 and the IL-1 pathway strongly suggest involvement of the inflammasome. Second, histologic studies demonstrated increased infiltration with innate immune cells, but not T, B, or dendritic cells in the peripheral joint and gut of patients with AS.56–58 Third, human AS and its experimental models have a predilection for sites exposed to either microbial or mechanical stress. The latter aspect has led to the concept that AS and other forms of spondyloarthritis are primarily characterized by inflammation of the so-called synovio-entheseal complex.59 In this perspective, it is interesting to note that the fibrocartilage protein versican, which could be released during mechanical stress and induces spondylitis in BALB/c mice, is also a ligand for the innate immune receptor TLR2. Formal proof that innate immune alterations may trigger AS remains, however, awaited.
Independently of the exact origin of the inflammation, it is clear that several proinflammatory cytokines are pivotal in the downstream effector mechanisms. TNF plays a crucial role in the disease process as evidenced by the successful introduction of TNF blockade for this disease. Accordingly, transgenic overexpression of TNF in mice leads not only to severe, destructive polyarthritis but also to sacroiliitis.60 However, it remains incompletely understood which cells are the main producers of TNF in AS, in which form (soluble vs. transmembrane) and through which receptor (TNFR1 or TNFR2) TNF exerts its pathogenic effects, and which are the main target cells. In line with the genetic data, experimental data suggest a role for TNFR1 signaling in stromal cells.61 Besides TNF, the genetic data and emerging data form functional studies on the unfolded protein response implicate IL-23 and thus possibly IL-17 as important cytokines in the pathogenesis of AS.62
Clinical trials targeting the IL-23/IL-17 pathway in AS are currently being performed.
Structural Remodeling and Ankylosis
A crucial and largely unexplained aspect of the pathogenesis of AS is the occurrence of structural remodeling and new bone formation, ultimately leading to ankylosis. Human and experimental studies have revealed three important concepts. First, the remodeling phenotype of AS cannot be explained by the absence of joint destruction because erosive disease is evidenced by histology and radiology and because the cellular and molecular machinery for cartilage and bone destruction is present and operative at the sites of disease.63,64
Second, structural remodeling and osteoproliferation are dependent on endochondral bone formation. This process is governed by several molecular pathways including bone morphogenetic protein (BMP) and Wnt signaling.65,66
There is emerging evidence that several inhibitors of the Wnt pathway are dysfunctional in human spondyloarthritis and that this correlates with new bone formation.67 Third, osteoproliferation is not critically dependent on inflammation because, despite clinical efficacy on signs and symptoms, TNF blockade fails to halt radiologic progression in AS and both processes can be uncoupled in experimental models.68,69
On the other hand, vertebral sites showing signs of inflammation at baseline have a higher risk for subsequent osteoproliferation on follow-up.70 The exact relationship between inflammation and osteoproliferation needs to be further clarified because it may have important consequences for treatment.
Pathology
Axial Skeleton
Detailed histopathologic studies of axial involvement in AS are limited owing to the inaccessibility of biopsy material of both SI joints and the spine. A controlled study of SI biopsies from AS patients at various stages of disease and controls showed cellular infiltration with lymphocytes, macrophages, and plasma cells in the synovium and subchondral marrow as the earliest features of disease.71 Later features include the development of pannus extending from both synovium and subchondral bone marrow, with erosion of articular cartilage and its replacement by granulation tissue. Osteoclast formation and erosion of subchondral bone account for the typical widening of the joint spondyloarthritisce seen on plain radiography. Enthesitis is also evident in later stages of disease at the insertion of the posterior capsule. Reparative changes include cartilage metaplasia at sites of active inflammation, followed by its calcification and then replacement by endochondral bone, leading to obliteration of the joint space by ankylosis. Para-articular changes include bone sclerosis and fat replacement of bone marrow. Regarding the spine, pathologic data are limited except for a number of recent studies of apophyseal joints. Immunohistologic analysis shows subchondral lymphocyte infiltrates with CD4+ and CD8+ T cells, together with hypervascularization and foci of CD68+ osteoclastic cells.72
Peripheral Skeleton
Regarding peripheral joint manifestations of AS, histopathologic studies have assessed surgical samples of affected hip joint, synovial biopsies of knee and ankle joint, and enthesitis. Involvement of the hips is characterized by subchondral granulation tissue and osteoclast formation in the femoral heads and acetabulum that is associated with degradation of overlying articular cartilage.73 Synovial studies have revealed that the type of inflammation is strongly different from that observed in RA, with increased vascularity, increased infiltration with innate immune cells, and absence of specific features of T and B cell autoimmunity.74–77
Interestingly, these studies revealed a similar pattern of inflammation in AS in comparison with other subtypes of spondyloarthritis.78
Entheseal involvement most frequently occurs at sites rich in fibrocartilage such as the Achilles tendon. Inflammation and chronic cellular infiltration of soft tissues are relatively sparse but may be extensive within the adjacent subchondral bone, particularly in B27+ individuals.79A comparative study of the subchondral marrow from knee and hip joint entheses showed that AS patients clearly differ from those with RA and osteoarthritis with respect to the frequency of marrow inflammation, infiltration with CD8+ T cells, and presence of hyperosteoclastic erosive lesions.
Clinical Manifestations
Skeletal Manifestations
Low Back Pain and Stiffness
Back pain is an extremely common symptom, occurring in up to 80% of the general population. Therefore it is important to note that back pain in AS and axial spondyloarthritis has special features that differentiate it from mechanical back pain80–82 (Table 75-6). In clinical practice inflammatory back pain is often not well recognized.83 Back pain is the most prevailing diagnostic feature of AS (Table 75-7).
Table 75-6 Aspects of Inflammatory Back Pain in Ankylosing Spondylitis and Axial Spondyloarthritis
Table 75-7 Diagnostic Features of Ankylosing Spondylitis
Chest Pain
With subsequent involvement of the thoracic spine (including costovertebral and costotransverse joints) and the occurrence of enthesitis at the costosternal and manubriosternal joints, patients may experience chest pain accentuated by coughing or sneezing, which is sometimes characterized as “pleuritic.” The chest pain is often associated with tenderness over the sternocostal or costosternal junctions. Mild to moderate reduction of chest expansion is often detectable in an early stage of AS. Chest pain occurs relatively often in HLA-B27+ relatives, even in the absence of radiographic evidence of sacroiliitis.84
Extraskeletal Manifestations
Eye Disease
Acute anterior uveitis or iridocyclitis is the most common extra-articular manifestation of AS, occurring in 25% to 30% of patients at some time during the course of the disease. There is no clear relationship between activity of the articular disease and this extra-articular manifestation. The onset of eye inflammation is usually acute and typically unilateral, but the attacks may alternate. The eye is red and painful, with visual impairment. Photophobia and increased lacrimation may be present. If the eye remains untreated or if treatment is delayed, posterior synechiae and glaucoma may develop. Most attacks subside in 4 to 8 weeks without sequelae if early treatment is provided. Acute anterior uveitis is more common in B27+ than B27− patients with AS.85 Relatives who have acute anterior uveitis seem to be at higher risk for AS. The calculated incidence of acute anterior uveitis in a Swiss family study was 89 attacks per 1000 patient-years for AS patients, but only 8 per 1000 person-years among healthy B27+ relatives.86
Cardiovascular Disease
Cardiac involvement may be clinically silent or may cause considerable problems. Manifestations of cardiac involvement include ascending aortitis, aortic valve incompetence, conduction abnormalities, cardiomegaly, and pericarditis. In rare situations, aortitis may precede other features of AS. Aortic incompetence was noted in 3.5% of patients who had the disease for 15 years and in 10% after 30 years.87 Inflammation and dilation of the aorta are the main causes of aortic valve incompetence. Cardiac conduction disturbances are seen with increasing frequency with the passage of time, occurring in 2.7% of those with disease of 15 years’ duration and in 8.5% after 30 years.87 Both aortic incompetence and cardiac conduction defects occur twice as often in patients with peripheral joint involvement. In AS the prevalence of myocardial infarction is increased (4.4% in AS patients compared with 1.2% in the general population in a Dutch study).88
Pulmonary Disease
Lung involvement is a rare and late manifestation of AS. It is characterized by slowly progressive fibrosis of the upper lobes of the lungs, appearing, on average, 2 decades after the onset of AS. Patients may complain of cough, dyspnea, and sometimes hemoptysis.89
High-resolution computed tomography (CT) may be helpful in detecting interstitial lung disease in patients with respiratory symptoms whose chest radiographs are normal.90 This imaging technique reveals a high prevalence of lung changes even among AS patients with early disease and without respiratory symptoms. The clinical significance of these findings is unknown. Long-term prospective studies need to be performed.91
Neurologic Involvement
As in RA, atlantoaxial joint subluxation, atlanto-occipital subluxation, and upward subluxation of the axis may occur in AS as a consequence of instability resulting from the inflammatory process. Spontaneous anterior atlantoaxial subluxation is a well-recognized complication in about 2% of patients and manifests with or without signs of spinal cord compression. It is observed more commonly in patients with spondylitis and peripheral arthritis than in those with exclusively axial involvement.92
The cauda equina syndrome is a rare but serious complication of long-standing AS. The syndrome affects lumbosacral nerve roots. This gives rise to pain and sensory loss, but frequently there are also urinary and bowel symptoms. Gradual onset of urinary and fecal incontinence, impotence, saddle anesthesia, and occasionally loss of ankle jerks occurs. Motor symptoms, if present, are usually mild. CT and MRI allow the accurate noninvasive diagnosis of this complication of AS.93 No compressive lesions exist. Arachnoiditis and arachnoid adhesions may be important in the pathogenesis.
Renal Involvement
IgA nephropathy has been reported in many patients with AS. These patients often have an elevated immunoglobulin (Ig)A level (93%) and renal impairment (27%) at presentation.94 Microscopic hematuria and proteinuria may occur in up to 35% of patients. The significance of these findings in terms of subsequent deterioration of renal function is unclear.95 Amyloidosis (secondary type) is a rare complication. Amyloid deposits detected through abdominal subcutaneous fat aspiration are not invariably associated with a poor renal prognosis.96
Osteoporosis
Osteopenia is seen in the early stages of AS.97 In patients with this disease, osteoporotic deformities of the thoracic spine contribute significantly to abnormal posture, particularly fixed hyperkyphosis.98 Radiographic damage to the cervical and lumbar spine, thoracic wedging, and disease activity are determinants of hyperkyphosis in AS.99 An increased occiput-to-wall distance is associated with vertebral fractures. The prevalence of symptomatic osteoporotic spinal fractures is increased in AS.100 Neurologic complications occur rather frequently, even after minor trauma.101 Proper assessment of bone density in the spine is difficult in the presence of syndesmophytes because they may give rise to falsely high values. The true fracture risk and complication rate in early and late disease and the relation to disease activity are not yet known. Currently, it is unclear whether any specific antiosteoporotic therapy to prevent spinal fractures is effective.
Physical Findings
Posture
After many years of progression in patients with severe disease, the entire spine may become increasingly stiff, with loss of normal posture from gradual loss of lumbar lordosis and the development of thoracic kyphosis.98,99 The abdomen becomes protuberant; breathing is primarily by diaphragmatic action. These typical deformities usually evolve after disease duration of 10 years or more.
Laboratory Tests
Generally, routine blood tests are not helpful. A normal erythrocyte sedimentation rate (ESR) or normal C-reactive protein (CRP) level does not exclude active disease. An elevated ESR or CRP is reported in up to 75% of patients, but it may not correlate with clinical disease activity.102 In an unselected patient population, an elevated ESR and CRP was present in 45% and 38%, respectively, of patients with spinal disease only, compared with 62% and 61%, respectively, of patients with peripheral arthritis with or without inflammatory bowel disease. Neither ESR nor CRP is superior in assessing disease activity.103 A mild normochromic anemia may be present in 15% of patients. Elevation of serum alkaline phosphatase (derived primarily from bone) is seen in some patients but is unrelated to disease activity or duration. Some elevation of serum IgA is frequent in AS. Its level correlates with acute-phase reactants. Active disease is associated with decreased lipid levels, particularly high-density lipoprotein cholesterol, resulting in a more atherogenic lipid profile.104
Imaging Studies
Conventional Radiography
The typical radiographic changes of AS are seen primarily in the axial skeleton, especially in the SI, discovertebral, apophyseal, costovertebral, and costotransverse joints. They evolve over many years, with the earliest, most consistent, and most characteristic findings seen in the SI joints. However, otherwise typical AS has been described in the absence of radiographic evidence of sacroiliitis.8 The radiographic findings of sacroiliitis are usually symmetric and consist of blurring of the subchondral bone plate, followed by erosions and sclerosis of the adjacent bone. The changes in the synovial portion of the joint (i.e., the lower two-thirds of the joint) result from inflammatory synovitis and osteitis of the adjacent subchondral bone.105 The cartilage covering the iliac side of the joint is much thinner than that covering the sacral side. Therefore the erosions and subchondral sclerosis are typically seen first and tend to be more prominent on the iliac side.
Ultimately, usually after several years, there may be complete bony ankylosis of the SI joints, with resolution of bony sclerosis. It is practical to grade radiographic sacroiliitis according to the New York criteria (Table 75-8).
Table 75-8 Grading of Sacroiliitis: New York Criteria
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|