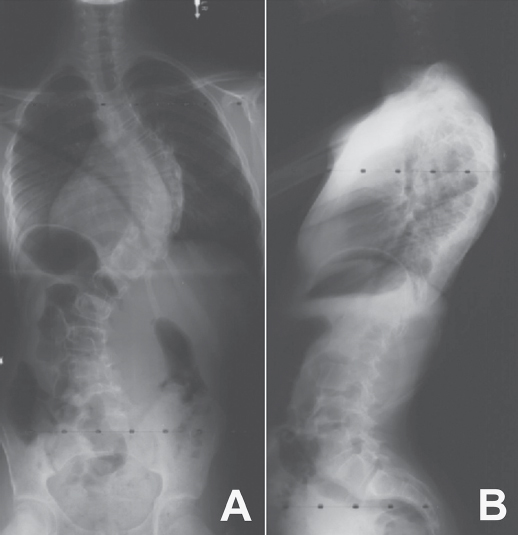
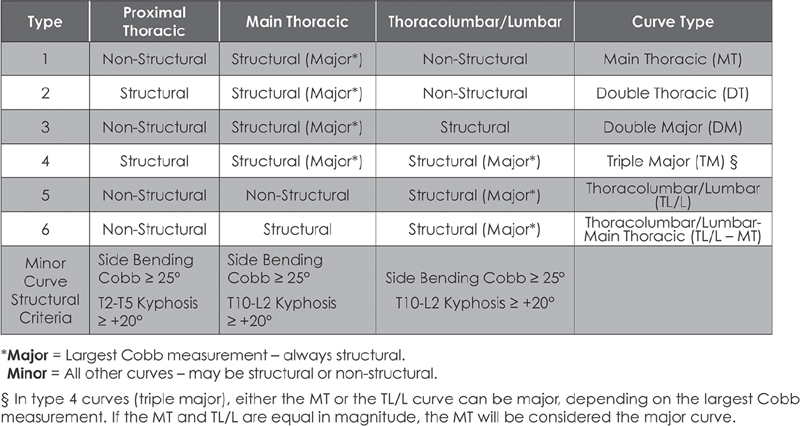
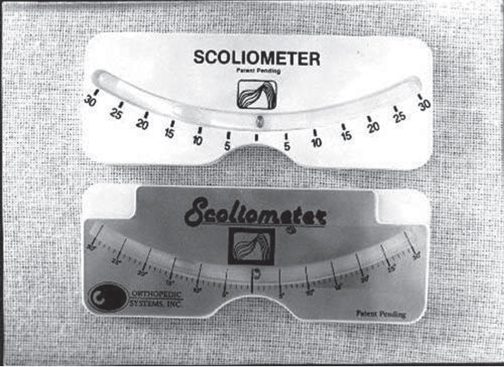
39 The Scoliosis Research Society (SRS) defines adolescent idiopathic scoliosis (AIS) as a lateral curvature of the spine having a Cobb angle measuring 10 degrees or greater on standing radiographs, and without any underlying neuromuscular disorders, in a patient between 10 and 18 years old. The most common type of curve involves a thoracic curve with an apex toward the right, and the condition is two times more common in girls than in boys. While coronal deformity is most commonly associated with AIS, this condition is a three-dimensional deformity caused by wedging of the vertebrae and intervertebral disks (coronal deformity), loss of thoracic kyphosis (sagittal deformity), and vertebral rotation (axial deformity). The major risk factors for curve progression include female gender, family history of severe scoliosis, future growth potential, and curve magnitude at the time of diagnosis (Fig. 39.1A,B). The etiology of AIS remains unclear, and ongoing research has revealed a complex and multifactorial pathophysiology. Platelet microstructure abnormalities, increased calmodulin, and decreased melatonin have been suggested to play a role in the development and progression of AIS; however, their mode of action and relationship to AIS remain unclear.1 The role of genetic factors in the development and progression of AIS has been demonstrated through pedigree and population studies, as well as advances in genetic testing. Twin concordance studies have demonstrated that monozygotic twins have a higher likelihood of both having scoliosis if one twin does (73%) than dizygotic twins have (36%). However, the specific mode of inheritance of AIS has not been resolved, and evidence has suggested autosomal dominant, X-linked dominant with variable penetrance/expression, and multiple gene/multifactorial inheritance with variable penetrance/expression.2 The future role of genetic testing in the management of AIS is undetermined; however, there are continued efforts to use genetics to predict the probability of curve progression to a severity requiring surgery. Currently, there are saliva-based genetic markers validated in white girls and boys, but not yet confirmed in Asians or African Americans, which are expected to personalize medical decisions and allow more effective care by lowering cost and requiring fewer unnecessary radiographs and brace applications.3,4 Fig. 39.1 Radiographs of an AIS patient. (A) Anteroposterior. (B) Lateral. Two well-known classification systems for adolescent idiopathic scoliosis are the King-Moe and Lenke classifications. In 1983, King and Moe introduced their classification system for AIS, describing five different curve types.5 The King-Moe classification has many shortcomings, which were recognized as surgical techniques and instrumentation evolved for the treatment of AIS. As a result, in 2001 Lenke et al. formulated a surgical classification system that was (1) more comprehensive, (2) two-dimensional with applicability to three-dimensional assessment, (3) treatment based, (4) able to separate specific curve types by objective radiographic criteria, (5) highly reliable, and (6) easily understandable to scoliosis surgeons.6 Although this classification has limitations with respect to reliability and may seem to be confusing to the infrequent user, it offers a more comprehensive radiographic evaluation for operative planning than previous classifications did (Fig. 39.2). Fig. 39.2 The Lenke classification system. (From O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. The Spinal Deformity Group Radiographic Measurement Manual. Memphis, TN: Medtronic Sofamor Danek; 2004. Reprinted with permission.) Patients most commonly present for evaluation of AIS as a result of school screening or body asymmetry noted by a parent. These body asymmetries consist of trunk shift, uneven shoulders, rib prominence, or breast or waist asymmetry. Despite these deformities, patients do not typically complain of pain or functional limitations. In severe curves, patients should be questioned for symptoms of pulmonary insufficiency. An appropriate growth and developmental history should be obtained, as well as when the curve was first noted, and if there is any evidence of progression. In some cases, old records or films are available and may be helpful. Any family history of severe scoliosis should be obtained as this increases the likelihood of a progressive curve. In females, the onset of menarche should be noted, as most surgeons believe the spine ceases significant growth within two years after the start of menarche. Physical examination of a patient with AIS should include a comprehensive neurological, musculoskeletal, and dermatologic evaluation to identify any potential etiologies for the underlying spinal deformity.9 An accurate height and weight should be recorded at each visit. In AIS the neurologic examination should be normal, and any abnormality, including asymmetry of the abdominal reflexes, may indicate an underlying intraspinal anomaly. The most common physical examination technique is the Adams forward bending test, which is performed by visually assessing chest wall asymmetry or “rib hump” as a standing patient bends forward at the waist to touch his or her toes, keeping the knees straight. The magnitude of the rib hump can be measured using a Scoliometer (Orthopaedic Systems, Inc., Union City, CA), with a reading greater than 5 degrees associated with a scoliosis of at least 10 degrees. Other findings to note include an elevation of one shoulder or a plumb line that falls to the side of the gluteal crease when suspended from the C7 spinous process. Side bending can be used to assess the flexibility of the curve clinically (Fig. 39.3). Standard radiographic evaluation consists of a scoliosis survey, including 36-inch posteroanterior (PA) and lateral radiographs. The magnitude of deformity should be measured using the Cobb angle. This angle is determined by drawing a line along the superior endplate (SEP) of the most cephalad vertebra in a curve where the SEP is still oriented toward the concavity of the curve. Then the inferior endplate of the most caudad vertebra still pointing toward the concavity of the curve is assessed, and the angle between the lines measured to obtain the Cobb angle. Constructing perpendicular lines to the endplate lines assists in measuring this angle. The surgeon should note the spinal levels involved, the direction of the curve apex (right or left), the degree of rotation of the curve (by assessing for asymmetry of the pedicles on the PA film), and estimation of skeletal maturity. The most commonly used radiographic method for estimating skeletal maturity is based on the appearance and fusion of the iliac apophysis, which is quantified from 0 to 5 using the Risser stages. Stage 0 occurs before ossification of the iliac apophysis, while stages 1–4 are sequential quarters of ossification from ventral to dorsal until the entire iliac wing is capped. Lastly, stage 5 occurs when the apophysis fuses to the body of the ilium. Pubescence is marked with the appearance of the iliac ossification center, and spinal growth is considered complete at Risser stage 4.9 Fig. 39.3 Scoliometer. (Manufactured by Mizuho OSI, Union City, CA.) Curve flexibility has become a crucial component of curve analysis and surgical decision making. Several techniques are commonly used to determine the rigidity of the curve(s) and choose fusion levels, which include the supine lateral bending, supine traction, push-prone, fulcrum bending radiographs, and intraoperative supine traction under general anesthesia.10 There is ongoing discussion on the optimal technique to predict the amount of postoperative correction, as well as anticipating the effect of postoperative correction on the curve above and below the level of fusion and on the overall spinopelvic balance. There is evidence that supine lateral bending radiographs may not correlate with results associated with modern spinal instrumentation.10,11 Depending on specific curve characteristics, computed tomography (CT) or magnetic resonance imaging (MRI) is occasionally useful to gain a better appreciation of the deformity or to assess associated intraspinal abnormalities. MRI should be considered if there are any unusual clinical symptoms of pain or radiculopathy, any abnormalities on the neurologic examination, rapid curve progression, and an atypical curve pattern, such as a thoracic curve with an apex toward the left, a short segment curve with sharp angulation, or absence of thoracic apical segment lordosis.12 There are three principal treatment options for AIS: observation, bracing, and surgical arthrodesis. The need for intervention (bracing or surgery) is determined by assessing the likelihood of curve progression, which can be estimated by the magnitude of the Cobb angle and the growth potential. Growth potential is determined by peak growth velocity, menarchal status, and the Risser sign. Because there is a high risk of progression, curves greater than 40 to 50 degrees are generally indications for arthrodesis (surgical fusion) in a growing child. Observation is warranted for idiopathic curves with a Cobb angle of less than 25 degrees. The SRS currently recommends initiation of brace treatment in skeletally immature patients who present with curves greater than 30 degrees on initial presentation or who progress greater than 10 degrees to a magnitude greater than 25 degrees.13 Braces are prescribed to be worn 18 to 23 hours a day, although evidence demonstrates the effectiveness of part-time (at least 12 hours per day) brace wear to address patient compliance issues.14 Brace wear is continued until skeletal growth is complete, as determined by unchanged height measured consecutively 6 months apart, Risser stage 4 (females) or 5 (males), or until 2 years after menarche in females. Generally, one should achieve ~ 50% curve correction with brace application. Failure to obtain this correction predicts a poor prognosis for successful bracing. Successful bracing will not improve the existing curve and is designed to halt progression of the curve and avoid surgical intervention. An alternative goal for bracing is to allow continued growth in a child with scoliosis who is likely to need surgery but still has significant remaining growth potential.15 A recent evidence-based review of the literature reported a 23% risk of needing surgery despite best efforts at bracing compared with 22% with observation.16 There are no level 1 evidence bracing studies currently in the literature, and the efficacy and effectiveness of brace treatment remain unproven and controversial. There is currently an ongoing level 1 study, BrAIST (Bracing in Adolescenet Idiopathic Scoliosis Trial), which will compare full-time and part-time bracing for preventing curve progression. Of those diagnosed with AIS, only ~ 10% eventually require surgical treatment. The general indications for surgical treatment in AIS include progressive curves greater than 40 to 45 degrees in skeletally immature patients, curve progression despite bracing, or occasionally an unacceptable cosmetic deformity. The principal goal of surgery is to correct a deformity that is likely to progress and cause functional limitations or cardiopulmonary deterioration. The most critical step in surgical planning is the selection of the approach and the levels to be included in the construct.15 If a curve is instrumented too short and does not include all levels of the major curve, there is a risk for the development of a progressive junctional deformity or “adding-on” phenomenon cephalad or caudal to the structural curve. When too many levels are included in a construct, this involves additional surgical time, increased instrumentation cost, and a small increased risk of nonunion. Also, overcorrection should be avoided to maintain overall global alignment and balance of the spinal column and prevent postoperative shoulder imbalance. Historically, severe and rigid curves often require a circumferential (360 degree) approach with an anterior spinal release, followed by posterior spinal fusion with instrumentation. There is good evidence to support a posterior-only procedure with the use of pedicle screws and Ponte-type soft-tissue or bony osteotomies, thereby reducing the need for separate procedures.17 Relative indications for a circumferential approach include curves more than 100 degrees and curves associated with hyperkyphosis (greater than 70 degrees in the thoracic spine or frank kyphosis in the lumbar spine). Also, patients with open triradiate cartilage are at risk for crankshaft phenomenon, which is progressive rotational deformity that occurs despite a solid posterior fusion, as a result of anterior spinal growth. While the absolute risk of crankshaft phenomenon appears to be less than 50%, curves in young patients may require anterior arthrodesis to prevent progressive deformity, although the three-dimensional control afforded by pedicle screws is thought to decrease this phenomenon. The natural history of untreated scoliosis, especially in curves > 50 degrees, is a slow, continued progression even after the cessation of growth. A recent prospective multicenter study has reported that preoperative bracing negatively impacts postoperative outcome after posterior spine fusion with instrumentation for AIS. Braced patients complained of more pain, were less active, and had lower SRS scores at 2 years after surgical correction.18 In another recent prospective multicenter study there was significant improvement in SRS scores at 2 years after surgical correction of AIS, with poor outcomes and less satisfaction related to pain and poor function preoperatively, and patients with less spinal appearance issues preoperatively (higher body mass index or Lenke 3 curves).19 Intermediate-term follow-up after posterior pedicle screw–only constructs for AIS have also reported outcomes with improvement in SRS scores, no neurologic or vascular complications, and maintained radiographic curve correction.20–22 There are various risks associated with the surgical correction of AIS. Although rare, the most concerning is the risk of paralysis or neurologic deficit, with the reported prevalence ranging from 0.3% to 1.4%.23 Neurophysiologic monitoring, using transcranial electric motor evoked potentials (MEPs) in addition to somatosensory evoked potentials (SSEPs), is recommended for early detection of an evolving or impending spinal cord deficit during surgical correction for AIS.24 The Stagnara wake-up test is a useful adjunct to neurophysiologic monitoring for the detection of neurologic spinal cord deficits.24 Pseudarthrosis or fusion failure remains a small risk (1–3% for AIS), but has decreased with the use of modern instrumentation and grafting procedures.20 The prevalence of nonneurologic postoperative complications following surgery for correction for AIS ranges from 5 to 15%.25 The factors significantly increasing the rate of complications include increased operative blood loss, prolonged posterior surgery time, and prolonged anesthesia time.25 Anterior surgery increases the risk of respiratory dysfunction in the postoperative period. The advances in correctly classifying adolescent idiopathic scoliosis and the development of modern instrumentation allowing advanced correction maneuvers, such as direct vertebral derotation, have changed our approach to the treatment of this condition.26 There still is controversy, however, in the areas of brace treatment (i.e., whether there are true benefits of being braced), school screening, and the ultimate cause of AIS. Although the knowledge gap has significantly decreased over the past 10 years, there are still strides to make in the understanding and treatment of AIS. 1. Kouwenhoven JW, Castelein RM. The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine 2008;33(26):2898–2908 PubMed 2. Heary RF, Madhavan K. Genetics of scoliosis. Neurosurgery 2008;63(3, Suppl):222–227 PubMed 3. Ogilvie J. Adolescent idiopathic scoliosis and genetic testing. Curr Opin Pediatr 2010; 22(1):67–70 PubMed 4. Ward K, Ogilvie J, Argyle V, et al. Polygenic inheritance of adolescent idiopathic scoliosis: a study of extended families in Utah. Am J Med Genet A 2010;152A (5):1178–1188 PubMed 5. King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am 1983;65(9):1302–1313 PubMed 6. Lenke LG, Betz RR, Clements D, et al. Curve prevalence of a new classification of operative adolescent idiopathic scoliosis: does classification correlate with treatment? Spine 2002; 27(6):604–611 PubMed 7. Fong DY, Lee CF, Cheung KM, et al. A meta-analysis of the clinical effectiveness of school scoliosis screening. Spine 2010;35(10):1061–1071 PubMed 8. Richards BS, Vitale M. SRS/AAOS position statement: Screening for idiopathic scoliosis in adolscents. Scoliosis Research Society. Reviewed Aug 2007. http://www.srs.org/professionals/positions/?id=62. Accessed Oct 8, 2010 9. Harrop JS, Birknes J, Shaffrey CI. Noninvasive measurement and screening techniques for spinal deformities. Neurosurgery 2008;63(3, Suppl):46–53 PubMed 10. Hamzaoglu A, Talu U, Tezer M, Mirzanli C, Domanic U, Goksan SB. Assessment of curve flexibility in adolescent idiopathic scoliosis. Spine 2005;30(14):1637–1642 PubMed 11. Cheung KM, Natarajan D, Samartzis D, Wong YW, Cheung WY, Luk KD. Predictability of the fulcrum bending radiograph in scoliosis correction with alternate-level pedicle screw fixation. J Bone Joint Surg Am 2010;92(1):169–176 PubMed 12. Davids JR, Chamberlin E, Blackhurst DW. Indications for magnetic resonance imaging in presumed adolescent idiopathic scoliosis. J Bone Joint Surg Am 2004;86-A (10):2187–2195 PubMed 13. Schiller JR, Thakur NA, Eberson CP. Brace management in adolescent idiopathic scoliosis. Clin Orthop Relat Res 2010;468(3):670–678 PubMed 14. Katz DE, Herring JA, Browne RH, Kelly DM, Birch JG. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am 2010;92(6):1343–1352 PubMed 15. Angevine PD, Deutsch H. Idiopathic scoliosis. Neurosurgery 2008;63(3, Suppl):86–93 PubMed 16. Dolan LA, Weinstein SL. Surgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence-based review. Spine 2007;32(19, Suppl):S91–S100 PubMed 17. Luhmann SJ, Lenke LG, Kim YJ, Bridwell KH, Schootman M. Thoracic adolescent idiopathic scoliosis curves between 70 degrees and 100 degrees: is anterior release necessary? Spine 2005;30(18):2061–2067 PubMed 18. Diab M, Sharkey M, Emans J, Lenke L, Oswald T, Sucato D; Spinal Deformity Study Group. Preoperative bracing affects postoperative outcome of posterior spine fusion with instrumentation for adolescent idiopathic scoliosis. Spine 2010;35(20):1876–1879 PubMed 19. Sanders JO, Carreon LY, Sucato DJ, Sturm PF, Diab M; Spinal Deformity Study Group. Pre-operative and perioperative factors effect on adolescent idiopathic scoliosis surgical outcomes. Spine 2010;35(20):1867–1871 PubMed 20. Lehman RA Jr, Lenke LG, Keeler KA, et al. Operative treatment of adolescent idiopathic scoliosis with posterior pedicle screw-only constructs: minimum three-year follow-up of one hundred fourteen cases. Spine 2008;33(14):1598–1604 PubMed 21. Cuartas E, Rasouli A, O’Brien M, Shufflebarger HL. Use of all-pedicle-screw constructs in the treatment of adolescent idiopathic scoliosis. J Am Acad Orthop Surg 2009;17(9): 550–561 PubMed 22. Mulpuri K, Perdios A, Reilly CW. Evidence-based medicine analysis of all pedicle screw constructs in adolescent idiopathic scoliosis. Spine 2007;32(19, Suppl):S109–S114 PubMed 23. Pahys JM, Guille JT, D’Andrea LP, Samdani AF, Beck J, Betz RR. Neurologic injury in the surgical treatment of idiopathic scoliosis: guidelines for assessment and management. J Am Acad Orthop Surg 2009;17(7):426–434 PubMed 24. Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am 2007;89(11):2440–2449 PubMed 25. Carreon LY, Puno RM, Lenke LG, et al. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Am 2007;89(11):2427–2432 PubMed 26. Lee SM, Suk SI, Chung ER. Direct vertebral rotation: a new technique of three-dimensional deformity correction with segmental pedicle screw fixation in adolescent idiopathic scoliosis. Spine 2004;29(3):343–349 PubMed Carreon LY, Puno RM, Lenke LG, et al. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Am 2007;89(11):2427–2432 PubMed In this prospective study of patients following surgery for AIS, the prevalence of nonneurologic complications was found to be 15.4%. Risk factors significantly increasing the rate of nonneurologic complications were history of renal disease, increased operative blood loss, prolonged posterior spinal surgery time, and total anesthesia time. Kim YJ, Lenke LG, Bridwell KH, Cho YS, Riew KD. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine 2004;29(3):333–342, discussion 342 PubMed Kim et al. describe the surgical technique for free-hand thoracic pedicle screw placement. The authors found the free-hand technique to be accurate, reliable, and safe after review of 394 patients with over 3204 thoracic pedicle screws placed over 10 years, and found no neurologic, vascular, or visceral complications. Lehman RA Jr, Lenke LG, Keeler KA, et al. Operative treatment of adolescent idiopathic scoliosis with posterior pedicle screw-only constructs: minimum three-year follow-up of one hundred fourteen cases. Spine 2008;33(14):1598–1604 PubMed Lehman et al. report the first intermediate-term findings following posterior spinal fusion with pedicle screw–only constructs for surgical correction of AIS. The study included 114 patients having a minimum of 3-year follow-up and found no neurologic, vascular, or visceral complications. The authors reported acceptable radiographic and clinical outcomes using posterior-only deformity correction with pedicle screws for AIS. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am 2001;83-A (8):1169–1181 PubMed This classic article presents the initial description of the Lenke classification for AIS. In 2001 Lenke et al. developed a new two-dimensional classification for AIS based on coronal and sagittal radiographs, and it was found to be more reliable than the King system. C-M-T, Charcot-Marie-Tooth
Adolescent Idiopathic Scoliosis
![]() Etiology
Etiology
![]() Classification
Classification
![]() Workup
Workup
Scoliosis Screening
History
Physical Examination
Spinal Imaging
![]() Treatment
Treatment
![]() Outcomes
Outcomes
![]() Complications
Complications
![]() Conclusion
Conclusion
References
Suggested Readings
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






