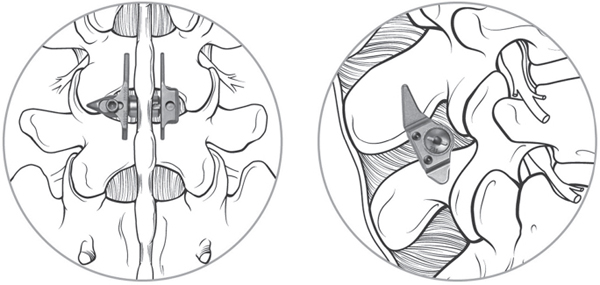
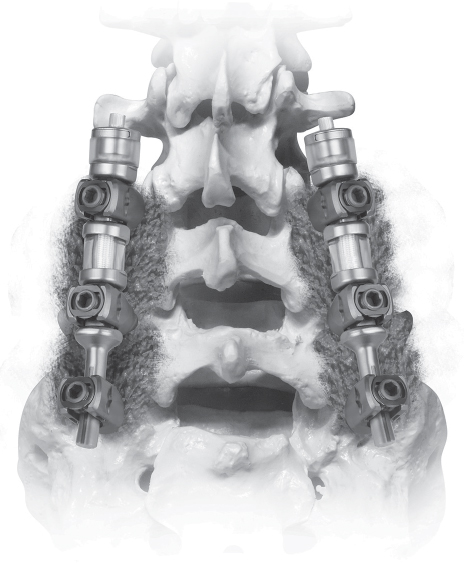
64 Normal function of the lumbar spine depends on segmental motion at each vertebral level. Although spinal arthrodesis is successful at treating multiple conditions, the loss of physiologic motion leads to increased loads adjacent to the fused levels. In an attempt to reconstruct the spine without a loss of segmental motion, several non-fusion devices have been developed. Proponents believe that such devices may preserve physiologic motion and thus decrease the risk of adjacent-segment degenerative disease. There are three categories of posterior non-fusion devices: interspinous process spacers, facet joint replacements, and pedicle screw–based flexible stabilization systems. The evaluation of patients being considered for posterior non-fusion surgery includes a standard history and physical examination. For patients being considered for an interspinous process spacer such as the X-Stop, a history of leg pain relieved with lumbar flexion is a prerequisite. Radiographs, including standing anteroposterior (AP), lateral, oblique, and dynamic (flexion-extension) views, are obtained as needed. Magnetic resonance imaging (MRI) studies are the most commonly used method to evaluate the neural elements and the condition of the intervertebral disks. Computed tomography (CT) scans provide better imaging of the facet joints and other bony structures for surgical planning. The concept of interspinous process spacer treatment is based on the observation that for many patients, symptoms of neurogenic claudication are relieved by flexion and worsened by extension. Interspinous process spacer implants are designed to block the vertebral segment from reaching terminal extension. In addition, one study suggests that by placing distraction at the stenotic levels, the areas of the canal and foramina are enlarged.1 A second biomechanical study showed unloading of the disk at the instrumented level and no change in disk pressure at adjacent levels.2 Of the interspinous process implants available, the best known in the United States is the X-Stop (Medtronic Sofamor Danek, Memphis, TN). This device consists of a titanium spacer placed between adjacent spinous processes, with small wings to prevent dislodgment (Fig. 64.1). The implant is designed to be placed under local anesthesia using a minimally invasive posterior incision. Two large trials of the X-Stop device have shown significant improvement in the symptoms of neurogenic claudication. One randomized controlled trial showed an improvement of 45% in mean symptom severity score and a 44% increase in physical function score on the Zurich Claudication Questionnaire at 2-year follow-up.3 A European study of 175 patients showed a decrease in visual analog scale (VAS) leg pain from 61.1 to 39.0 (p < 0.005), which was maintained at 2 years postoperatively. Mean Oswestry Disability Index (ODI) measurement decreased from 32.6 to 20.3 at 2 years postoperatively.4 Fig. 64.1 The X-Stop is a titanium spacer with two wings, implanted between the spinous processes. (Used with permission from Medtronic Inc., Memphis, TN.) Facet joint replacements are designed to treat lumbar stenosis due to facet hypertrophy. The Total Facet Arthroplasty System (TFAS; Archus Orthopedics, Redmond, WA) and ACADIA Facet Replacement System (Facet Solutions, Hopkinton, MA) are examples of such devices. Biomechanical studies have shown that kinematics at both the instrumented and adjacent levels are similar to those of the intact spine with facet arthroplasty systems.5 As of this writing, the TFAS and ACADIA systems are in phase III trials for FDA approval. No clinical outcomes data are yet available. Pedicle screw–based soft stabilization systems purportedly decrease pathologic motion of the affected spine segments while retaining physiologic motion. These devices use pedicle screws as anchors attached to semirigid intercalary cords. The Graf ligament was the first known device in this category. Since its inception, several similar devices have been created. Among these are the Dynesys (Zimmer Spine, Minneapolis, MN) and the Transition (Globus Medical, Audubon, PA). The Dynesys device is currently the best-known of the pedicle screw–based non-fusion systems and the best-studied to date. Both devices employ titanium pedicle screws connected with elastic polyethylene terephthalate (PET) cords inserted into polycarbonate urethane (PCU) spacers. The spacers can be adjusted to allow compression or distraction between segments. (Fig. 64.2) Extensive biomechanical testing of both the Dynesys and Transition devices has been performed as a part of the FDA approval process. The Dynesys has been shown to restrict range of motion in all planes significantly, although restriction in axial rotation was only 13% compared with the intact state.6 In another in vitro study, the Dynesys system was shown to increase peak facet loads in flexion and lateral bending, but not in axial rotation or extension.7 The Dynesys has been used since 1994 in Europe in more than 15,000 cases. One prospective trial with 83 patients employed the Dynesys device as treatment for “lumbar instability conditions” due to degenerative disk disease, degenerative spondylolithesis, or disk herniations.8 At 38-month follow-up, mean pain and function scores improved, but seven patients required surgery for adjacent-level degeneration. In addition, eight patients had radiographic screw loosening, and three required revision surgery for persistent pain. Fig. 64.2 The Transition system consists of titanium pedicle screws with elastic spacers. (Used with permission from Globus Medical, Audubon, PA.)
Posterior Lumbar Non-Fusion Devices
![]() Classification
Classification
![]() Workup
Workup
History and Physical Examination
Spinal Imaging
![]() Interspinous Process Implants
Interspinous Process Implants
![]() Facet Replacements
Facet Replacements
![]() Pedicle Screw–Based Soft Stabilization Systems
Pedicle Screw–Based Soft Stabilization Systems
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






