57 Acute Phase Reactants and the Concept of Inflammation
The concept of inflammation itself has evolved over thousands of years; Celsus first described the cardinal signs of redness, swelling, heat, and pain in the 1st century ad. The fifth sign, loss of function, is often attributed to Galen’s work during the 2nd century.1 Since that time, countless advances have been made in the understanding of inflammation, from histologic findings of inflammatory cells within tissues, to the discovery of hematologic and soluble mediators such as cytokines and complement. Also, the molecular signaling pathways that drive both its protective effects and the inappropriate injurious responses have been recently elucidated.
With increasing insight into mechanisms of the inflammatory response has come an appreciation of its significant complexity. Molecular and microscopic processes are different during acute and chronic stages, and diverse responses are induced by various types of exogenous and endogenous stimuli (e.g., bacteria, viruses, parasites, crystals, allergens, ischemia). More recently, the role of the milieu of the inflammatory response, especially at the level of the vascular endothelium, has taken on considerable importance.2 Furthermore, inherent redundancies of functions have been noted, as have interactions between the mediators of inflammation that allow for a broad, effective response, but these redundancies make it difficult to understand the pathways of inflammation in a linear fashion. These intricacies have resulted in a vague definition and on continued reliance on the final downstream macroscopic cardinal signs to tie together all of the ongoing processes. It is these same intricacies that have made evaluation of inflammation through laboratory tests imprecise.
Basic hematologic abnormalities may give clues to the presence of inflammation, but different patterns are often associated with underlying causes and are not universally found. Leukocytosis can be seen in infections, acute crystal diseases, and some autoimmune disorders such as adult Still’s disease. Anemia is often associated with certain diseases that cause chronic inflammation, such as rheumatoid arthritis (RA). Reactive thrombocytosis occurs secondary to the release of cytokines after an inciting infectious or inflammatory event, and the role of platelets and platelet-derived mediators in stimulating inflammation has been described at the molecular level.3
Acute Phase Response
Within minutes of tissue injury, activation of the innate immune system induces cytokine production that results in a multisystem acute phase response involving the liver, vascular system, bone marrow, and central nervous system.4,5 Many elements of the reaction can be regarded as part of the innate response and are defensive or adaptive in nature.6 Mouse studies have shown that up to 7% of the regulatable gene pool undergoes significant changes in expression during inflammation, and that induction of liver acute phase genes is mediated by the transcription factor signal transducer and activator of transcription 3 (STAT3).7–9
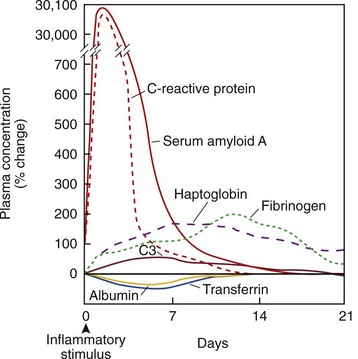
Although the acute phase response can trigger numerous neuroendocrine, hematopoietic, and metabolic effects, it is the changes in plasma proteins synthesized by hepatocytes that are monitored as signs of underlying inflammation (Tables 57-1 and 57-2). An acute phase protein is one whose plasma concentration changes from baseline by at least 25% during inflammation; responses vary in terms of concentration and kinetics (Figure 57-1).10 CRP and serum amyloid A (SAA) levels increase more than 1000-fold during acute infection, and peak at 2 to 3 days. Concentrations of other proteins peak at longer periods and can range from a 50% increase in complement and ceruloplasmin, to a several-fold amplification in haptoglobin, fibrinogen, α1-proteinase inhibitor, and α1-acid glycoprotein. Other proteins are negative acute phase proteins whose concentrations fall during the inflammatory response. These include antithrombin III, protein S, prealbumin, albumin, transferrin, and apolipoprotein A-I.4,5
Table 57-1 Human Acute Phase Proteins
| Patients Whose Plasma Concentrations Increase |
| Complement System |
| Coagulation and Fibrinolytic System |
| Antiproteases |
| Transport Proteins |
| Participants in Inflammatory Responses |
| Others |
| Proteins Whose Plasma Concentrations Decrease |
HS, Heremans-Schmid.
From Gabay C, Kushner I: Acte-phase proteins and other systemic responses to inflammation, N Engl J Med 340:448–454, 1999.
Table 57-2 Other Acute Phase Phenomena
| Neuroendocrine Changes |
| Hematopoeitic Changes |
| Metabolic Changes |
| Hepatic Changes |
| Changes in Nonprotein Plasma Constituents |
From Gabay C, Kushner I: Acte-phase proteins and other systemic responses to inflammation, N Engl J Med 340:448–454, 1999.
Hepatic stimulation of acute phase proteins is induced by cytokines released by activated monocytes, macrophages, neutrophils, natural killer (NK) cells, and endothelial cells acting at the front lines of the inflammatory response. The main cytokine influencing the liver is interleukin (IL)-6, once called the “hepatocyte-stimulating factor.” It likely mediates protein expression via the Janus-activated kinase (JAK) and STAT3 pathways, as well as C/EBP family members and Rel proteins (nuclear factor κB [NFκB]).9,11 During initial stages, IL-1 and tumor necrosis factor (TNF) synergize with IL-6 and trigger further IL-6 production, but their roles are limited.12 The soluble IL-6 receptor amplifies IL-6 effects both locally and systemically. IL-6 also performs a protective role during disease, inducing the expression of an IL-1 receptor antagonist.13
Acute phase protein levels are not uniform in their expression; this is likely related to the underlying pathophysiologic state and is regulated by different combinations and interactions of cytokines.5 The roles of the acute phase proteins themselves will be discussed throughout the chapter but have been found to include direct involvement in host defense by activation of the complement, proteinase inhibition, and antioxidant activity.14 However, some of the described in vitro effects of proteins may not be relevant in vivo.
Erythrocyte Sedimentation Rate
Although ESR is an indirect screen for elevated concentrations of acute phase proteins, it has been the most widely used marker of inflammation for almost a century. Measurement of ESR is performed when blood is placed in a vertical tube and the rate of fall of erythrocytes is measured. The ancient Greeks recognized increased red blood cell (RBC) sedimentation as a way to detect “bad bodily humors,” but our modern understanding and use of RBC sedimentation as a test date back to the German scholar Fahraeus in 1918.15 He determined that certain plasma proteins, especially fibrinogen, are able to lower the electrostatic charge on RBC surfaces so they can aggregate, form rouleaux, and fall faster.
Several factors are involved in acceleration of ESR. Asymmetric plasma proteins such as fibrinogen and, to a lesser extent, alpha2, beta, and gamma globulins decrease the negative charge of erythrocytes (zeta potential) that prevents rouleaux formation. Red cell factors also play a role in that changes in plasma ratios in anemic states also favor rouleaux. However, microcytosis, polycythemia, and abnormally shaped RBCs (e.g., sickle cells, spherocytes) hinder aggregation and lower the ESR.16 Conditions that elevate fibrinogen, even if they are not necessarily considered inflammatory, can raise ESR. These include pregnancy, diabetes, end-stage renal disease, and heart disease. Major increases in the concentration of a single molecular species, such as a monoclonal immunoglobulin in multiple myeloma, also cause increased sedimentation.17 The ESR is elevated in obesity, as is CRP, presumably as a result of IL-6 secretion by adipocytes.18 Factors such as glucocorticoids, cryoglobulins, hypofibrinogenemia, and hyperviscosity have been shown to lower the value.4 The physiochemical dynamics that allows for sedimentation has been a continued source of debate, with disparate models presented to explain how proteins on cell surfaces interact to cause RBC aggregation.19
Although novel and rapid tests for ESR have proved promising, the International Committee for Standardization in Hematology continues to recommend the Westergren technique of testing anticoagulated blood.20,21 The usual accepted upper limits of normal are 15 mm/hr for males and 20 mm/hr for females; however, the ESR increases with age and varies by race, calling the reliability of the test into question. A simple formula for calculating the upper limit of normal ESR at any age has been used regularly: In men, age in years divided by 2; in women, 10 plus age in years divided by 2. Despite the ability to control for age, other limitations of the test have been noted and are listed in Table 57-3. The relative virtues of CRP determination have diminished some of the importance of ESR, but it remains an easy, inexpensive test with a wealth of background literature. Therefore the sedimentation rate will continue to play a prominent role in clinical practice.
Table 57-3 Comparison of Erythrocyte Sedimentation Rate and C-Reactive Protein
| Erythrocyte Sedimentation Rate | C-Reactive Protein | |
|---|---|---|
| Advantages | ||
| Disadvantages |
C-Reactive Protein
C-reactive protein is an acute phase protein whose serum concentration reflects ongoing inflammation better than other tests in most, but not all, diseases.22 CRP was identified in 1930, when sera obtained from patients with Streptococcus pneumonia infection were found to contain a protein that could bind to the “C” polysaccharide of the bacterial cell wall. This protein circulates as a 115-kD pentamer of noncovalently linked 23-kD subunits, which has been highly conserved over hundreds of millions of years of evolution. In contrast to immunoglobulins and complement components, CRP deficiency in humans has not been described. Genome-wide associated studies performed recently have shown that at least seven distinct loci are involved in the basal expression of CRP,23–25 which is upregulated upon stimulation by the transcription factors C/EBP and Rel.26 It is present in trace concentrations in the plasma of all humans (roughly 1 mg/L, with higher concentrations in women and the elderly). Plasma C-reactive protein is synthesized by hepatocytes, although other sites of local production and possibly minimal secretion have been suggested.
The precise function of CRP is unknown and may be varied, but it exhibits important recognition and activation capabilities, and it binds to numerous ligands.27 CRP recognizes phosphocholine, phospholipids, fibronectin, chromatin, and histones, all of which are exposed at sites of tissue damage and by apoptotic cells; CRP may target them for clearance.28 C-reactive protein bridges the gap between innate and adaptive immunity by activating the classical complement pathway and interacting with cells of the immune system through binding of Fcγ receptors.29,30 CRP induces inflammatory cytokines, tissue factors, and shedding of the IL-6 receptor, all of which result in a complement-dependent increase in tissue damage.28 Other CRP functions are anti-inflammatory, including promoting the noninflammatory clearance of apoptotic cells and preventing neutrophil adhesion to the endothelium.31,32 Thus CRP may play many pathophysiologic roles during the course of the inflammatory process.14,33
After an acute inflammatory stimulus, CRP concentration increases rapidly and peaks at 2 to 3 days at levels that reflect the extent of tissue injury. If the stimulus has been removed, serum CRP levels drop rapidly, with a half-life of roughly 19 hours.34 Persistent elevations in CRP are seen in chronic inflammatory states such as active RA, pulmonary tuberculosis, or extensive malignant disease.
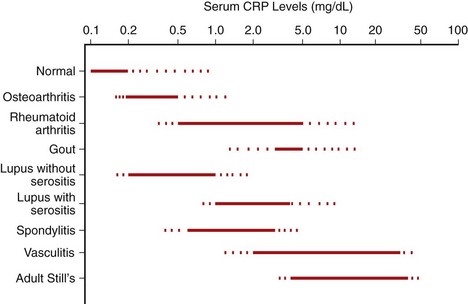
Immunoassays and laser nephelometry are used at modest cost to quantify serum CRP levels. Most healthy adults have levels less than 0.3 mg/dL. The significance of minor elevations in CRP is being debated and will be discussed subsequently. However, usual methods of CRP determination are less precise at concentrations in the range of 0.3 to 1 mg/dL, so high-sensitivity (hs)CRP methods are used to accurately measure these levels. Generally, concentrations greater than 1 mg/dL reflect clinically significant inflammatory disease.35,36 Concentrations of 1 to 10 mg/dL can be considered to represent moderate increases, and concentrations greater than 10 mg/dL show marked increases. Most patients with extremely high levels (e.g., >15 mg/dL) have bacterial infection; one study found that in patients with CRP concentrations greater than 50 mg/dL, infection was present in 88% of subjects.37 Clinical conditions associated with varying degrees of elevation of CRP are listed in Table 57-4, and the range of CRP concentrations in many rheumatologic diseases is shown in Figure 57-2.
Table 57-4 Conditions Associated with Elevated C-Reactive Protein Levels
| Normal or Minor Elevation (<1 mg/dL) | Moderate Elevation (1-10 mg/dL) | Marked Elevation (>10 mg/dL) |
|---|---|---|
Several limitations associated with the use of C-reactive protein measurement must be acknowledged. No uniformity in reporting concentrations has been noted between laboratories, and values can be conveyed in mg/L, µg/mL, or mg/dL. Similar to ESR, population studies show a skewed, rather than Gaussian, distribution, leaving parametric statistical tests inappropriate for interpretation of CRP data. Population differences in CRP levels in the United States have been reported between sexes and among racial groups. This is presumably also prevalent as an issue in practice in other populations globally. Elevation of C-reactive protein in the elderly may represent age-related disorders whose pathogenesis may involve low-grade inflammation, complicating the issue of what levels are considered normal.38
Serum Amyloid A
Serum amyloid A (SAA) consists of a circulating family of proteins produced by hepatocytes, adipocytes, macrophages, and fibroblast-like synoviocytes. Although some types are expressed constitutively, the hepatic production of SAA is responsible for an acute phase increase in plasma concentration—up to 1000-fold within 2 days of the inflammatory stimulus.39 SAA is the fibrillar component of amyloid deposits in secondary amyloidosis and is tightly associated with high-density lipoprotein (HDL).40
The function of SAA has not been fully defined, although it is generally considered proinflammatory; it likely acts in part through Toll-like receptor (TLR)2 activation of the NFκB pathway and binding to the G protein–coupled formyl peptide receptor like-1 (FPRL1).39 These actions induce a variety of cytokines, act as a chemotactic for leukocytes, and stimulate angiogenesis, tissue factor, and matrix metalloproteinase expression.41–44 SAA may have a direct role in immunity, acting as an opsonin for gram-negative bacteria and showing antiviral properties in vitro.45,46 It also has a well-identified role in cholesterol export from cells during inflammatory conditions.47,48
Numerous clinical associations with SAA have been described. Studies have shown correlation with disease activity in a number of inflammatory disorders, possibly more so than with ESR and CRP.49 Serum concentrations of SAA secreted by adipocytes correlate with body mass index and may provide a link between obesity and its comorbidities.50 The normal level of SAA in healthy adults is less than 10 mg/L, with a median value of 3 mg/L in a European population.51 The test could be useful in those at risk for secondary amyloidosis, whose probability of survival was higher than 95% after 6 years for individuals whose median SAA was less than 10 mg/L, but 40% if median SAA remained above 10 mg/L.52 However, reliable testing for acute phase SAA is not yet widely available, and data about levels expected in disease are limited.
Other Acute Phase Proteins
Measurement of other acute phase proteins has been of limited value clinically, because their responses to tissue injury are often slower, and the magnitude of concentration change is smaller than with CRP and SAA. Serum ferritin is moderately increased, triggered by cytokines such as IL-1, IL-6, IL-18, and TNF. Levels are frequently high in adult-onset Still’s disease and systemic lupus erythematosus (SLE), and they correlate with disease activity.53,54 Hepcidin, a liver-derived antimicrobial peptide and regulator of iron homeostasis, is induced by inflammation, and by IL-6 in particular.55 Its levels rise in parallel with ferritin, and it is important in the development of anemia of chronic disease, acting as a negative regulator of iron absorption and macrophage iron release teleologically, to deprive microbes of iron.56 Transferrin, which binds and transports iron, is a negative acute phase protein.
The prohormone of calcitonin, procalcitonin, is produced by many cell types during severe infection and has been used as a marker to distinguish bacterial infection from other types of inflammatory processes, with some caveats.57 Apolipoprotein a-I, the principal protein constituent of HDL, is another negative acute phase protein. In chronic inflammatory diseases such as RA and SLE, decreased levels may contribute to increased risk of thrombotic events.58,59 Serum albumin and prealbumin (transthyretin) are also negative acute phase proteins, although their measurement has not been shown to be more helpful in diagnosis or prognosis than standard tests.60 Serum complement fractions become depressed when the system is activated in certain autoimmune disorders but otherwise rise during the acute phase response.
Cytokines
Although not acute phase proteins in the classic sense, cytokines display the most striking acute phase behavior of any circulating proteins. IL-6 responds dramatically to tissue injury, with concentration changes that are faster and greater than those of CRP or SAA. Acute inflammation and chronic inflammation have been associated with increases in IL-6, and serum levels of this cytokine have been correlated with the severity and course of disease in RA, juvenile arthritis, ankylosing spondylitis, and polymyalgia rheumatica (PMR).61,62 It is also more sensitive than ESR for detecting disease activity in giant cell arteritis (GCA).63 IL-6 levels may be useful in monitoring inflammation if hepatocytes are damaged to the point of not being able to synthesize acute phase proteins.4 The importance of cytokines such as TNF and IL-1 has been inferred by the successful reduction of inflammation by their therapeutic inhibitors. Certain diseases, such as the TNF receptor–associated periodic syndrome (TRAPS) and the autoinflammatory syndromes that involve mutations of the inflammasome controlling IL-1, point to the importance of these cytokines.
Increased levels of several other cytokines and circulating cytokine receptors have been associated with inflammation or disease activity as well (Table 57-5).63,64 Different patterns of cytokine responses have been reported in different diseases, suggesting that cytokine determinations are potentially useful clinically.65,66 However, their quantitation presents several problems related to their short plasma half-lives, the presence of blocking factors and natural inhibitors, and other technical considerations.67 At present, high costs, limited availability, and absence of standardization discourage measurement of plasma cytokines and their receptors in clinical practice.
Table 57-5 Products of Inflammatory, Endothelial, and Resident Target Tissue Cells/Matrix
| Cytokines and Related Molecules | Products of Inflammatory and Endothelial Cells |
|---|---|
| Cytokines: | Calprotectin |
| IL-1 | von Willebrand factor |
| IL-6 | Soluble adhesion molecules (e.g., sVCAM and sE-selectin) |
| IL-12 | Hyaluronic acid |
| Interferon-α | Collagen and aggrecan degradation products |
| Tumor necrosis factor | Osteocalcin |
| Granulocyte-macrophage colony-stimulating factor | |
| IL-1 receptor antagonist |
IL, interleukin.
Acute Phase Reactants in the Management of Rheumatic Diseases
Rheumatoid Arthritis
ESR and CRP cannot be used in the diagnosis of RA because 45% of patients may have normal serum levels at presentation,68 although these values represent part of the diagnostic syndrome or classification criteria sets. These tests are more appropriately applied in RA for monitoring disease activity and response to therapy. Although ESR traditionally has been more widely used for these purposes, many studies have suggested that CRP levels correlate better with disease activity.69 Some recent reports state that CRP levels may overestimate disease response compared with ESR; others claim that differences between the two are minimal.69–71 The existence of patients with depressed CRP concentrations caused by carrying low-CRP–associated genetic variants must be taken into account when this test is used universally.72 Matrix metalloproteinase (MMP)-3, pro-MMP-3, and soluble E-selectin have also been proposed as markers for RA disease activity; their measurements correlate with CRP levels but do not provide more information than is provided by standard tests.73–75
CRP levels average 2 to 3 mg/dL in adult RA patients with moderate disease activity.76 However, variation is considerable: At least 5% to 10% of patients have values in the normal range, whereas a few patients with severe disease activity have levels greater than 10 mg/dL. ESR values have been found to remain stable over the years.77 ESR and CRP have long been used to follow the response to therapy; in general, effective disease-modifying antirheumatic drug therapy decreases CRP by about 40%. Inhibition of joint damage by these agents usually is accompanied by marked improvement in acute phase reactants. Progression of joint damage can occur while the patient is on therapy, however, despite decreases in ESR and CRP.78 Even more striking improvement has been seen with biologic agents introduced since the 1990s, providing objective laboratory support for the encouraging clinical responses observed. In early reports of anti-TNF therapy, CRP and SAA levels declined by 75% and 85% in about 1 week.79 Treatment with abatacept, the T cell CD80/CD86:CD28 co-stimulation modulator, resulted in significant decreases in CRP at both 90 and 360 days of therapy.80 In one study, failure to suppress CRP levels 2 weeks after initiation of infliximab therapy identified most patients who would prove to be clinical nonresponders after 12 weeks.81 In contrast to traditional disease-modifying antirheumatic drugs, TNF inhibitors have been found to inhibit joint damage even while clinical activity, reflected by CRP levels, remains high.82 Tocilizumab, a human IL-6 receptor antibody, improves RA by inhibiting effects of the cytokine. However, owing to its mechanism of action, inflammatory markers such as ESR and CRP drop to negative values, so that tracking them may not reflect the actual effect of the drug; care must be taken when monitoring disease activity during tocilizumab therapy.83,84
ESR and CRP also have value as prognostic indicators in RA. Elevated acute phase reactant levels are associated with early synovitis and erosions as detected by magnetic resonance imaging, with inflammatory cellular infiltrates in synovium, and with osteoclastic activation and reduced bone mineral density.85–87 CRP predicts radiographic progression, as do ESR and the matrix metalloproteinases MMP-3 and MMP-1.73,74,88–90 Finally, and perhaps most important, acute phase reactants correlate with work disability on long-term follow-up and predict progression to major joint replacement.91,92 As in the normal population, CRP levels are associated with death from cardiovascular disease.93 In RA patients who developed heart failure, ESR was higher during the 6-month period immediately preceding the onset of heart failure than earlier in their course.94
Serum or synovial fluid levels of many other tissue products (see Table 57-5) have been correlated with clinical measures of disease activity, severity, and radiographic damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree