LAWRENCE OSHER, ROCCO PETROZZI, AND ROBERT A. CHRISTMAN
GENERAL CONSIDERATONS
Primary bone tumor and tumorlike conditions rarely occur in the foot, with most reports ranging from approximately 2% to 3.5%. Studies generally concur that the phalanges and metatarsal bones are the most frequently involved pedal sites. Likewise, neoplasms of the midfoot bones are decidedly rare, leaving the talus and calcaneus as the second most common site behind the small tubular forefoot bones.
Looking at the entire skeletal system, a given bone tumor, when diagnosed, is approximately 4 times more likely to be benign versus malignant. When encountered, a given bone tumor is approximately 95 times more likely to be metastatic spread than a primary lesion. In addition, as a general rule, most metastatic lesions tend not to occur distal to the knee. If one adds all of this together, it is easy to see why the majority of pedal bone tumors encountered are benign.
Many practitioners are of the opinion that enchondroma is the most commonly encountered tumor of the foot, based on a well-known study by Coley and Higinbotham.1 However, the frequency of pedal enchondroma in the small tubular bones is probably overestimated, as this lesion is far more commonly encountered in the hand. For example, a number of more recent studies indicate that lesions such as osteochondroma and chondroblastoma are more frequent encounters. Of the primary malignant bone tumors, Ewing sarcoma is most common in younger age groups and chondrosarcoma appears the most frequent in adults.
As noted earlier, metastases are not common distal to the knee. When acrometastatic lesions occur in the foot, they are most frequently secondary to lung carcinoma or an aggressive visceral tumor. Lung (and some visceral) tumors may demonstrate symptomatic periostitis as a manifestation of hypertrophic pulmonary osteoarthropathy. When present, periostitis can commonly extend to the ankles and present with pain and swelling. However, foot involvement with hypertrophic osteoarthropathy is rare. Tumors with metastatic extension to the vertebral bodies are also known for the ability to extend distal to the knee (theoretically via the Batson vertebral plexus).
In long bones of the extremities, metastatic lesions may be lytic, blastic, or, more frequently, mixed lytic and blastic, with a propensity to demonstrate moth-eaten destruction. Periosteal reactions are variable, and may or may not comprise part of the overall picture. Acrometastasis is more likely to manifest gross lysis without periostitis. When an acrosclerotic lesion occurs, the differential diagnosis should include underlying conditions such as systemic lupus erythematosus, seronegative arthritis, and intraosseous sarcoidosis.
As a general rule, radiography is best used for diagnosis and differential diagnosis of a bone tumor, whereas staging is performed with computed tomography (CT) or magnetic resonance imaging (MRI). The cardinal radiographic characteristics with respect to patterns of internal bony lysis, periosteal reaction, cortical erosion, and matrix evaluation are used to assess bone tumors. Beyond this, probabilities in diagnosis frequency rest on epidemiologic data such as patient age and specific tumor location.
Clinical examination generally yields the nonspecific finding of pain, which often begins insidiously with an intermittent character. Duration of pain can be a clue as to the aggressiveness of the lesion, and progression to a severe, constant pain can occur with the more aggressive neoplasms or pathologic fracture. Other findings may include swelling, distension, and/or palpable mass; variable inflammation and local venous dilation; effusion into an adjacent joint; and occasional constitutional symptoms.
SYSTEMATIC EVALUATION OF SOLITARY BONE LESIONS
Solitary bone lesions are occasionally encountered in the foot and distal leg. Fortunately, most of these lesions are benign. Examples include fibrous cortical defect (FCD) and nonossifying fibroma (NOF), solitary bone cyst, enchondroma, and bone island. Some lesions have characteristic radiographic features; however, many appear similar to one another or only demonstrate subtle differences, and it may be impossible to distinguish between them by radiography alone. Determining the epicenter of the lesion and preferential direction of spread are often important discriminating clues. Unfortunately, much of this information cannot be determined when bone tumors are encountered in the small tubular bones of the foot as most, if not all, of the bone is involved by the time of discovery. On the other hand, early or incidental detection of benign lesions in small tubular bones tends to recapitulate behavior in extremity long bones.
An efficient, reliable approach is needed to assess these lesions. The radiographic features must first be recognized. These features can be used not only as diagnostic clues but also for determining the growth rate or aggressiveness of the lesion. A list of potential differential diagnoses can then be formulated based on these radiographic findings.
| Diagnostic Indicators for Evaluating the Solitary Bone Lesion |
Radiographic Clues
1. Destructive patterna
2. Size and shapea
3. Cortical involvementa
4. Periosteal reactiona
5. Anatomic position (transverse and longitudinal planes)
6. Skeletal location
7. Trabeculation
8. Matrix production
Nonradiologic Clues
1. Clinical course
2. Age of patient
aFeatures useful for determining a lesion’s growth rate or aggressiveness.
Ten diagnostic clues are used for assessing a solitary bone lesion (Table 20-1). Eight of these clues are radiographic features, and, of these, four are valuable for determining the aggressiveness or growth rate of the lesion. Two nonradiologic diagnostic clues complete the initial assessment: the patient’s age and clinical course. These ten criteria (eight radiographic and two clinical) should be considered when evaluating any solitary bone lesion. After these data are collected, differential diagnoses are determined.
Destructive Pattern
Three types of destructive (lytic) patterns have been described in the literature pertaining to solitary bone lesions: geographic, moth-eaten, and permeative (Figure 20-1).2 The term geographic implies large area, and lesions are typically 1 cm or greater in size. Geographic lytic lesions are often referred to as “cystic.” A sharply outlined lesion with a narrow zone of transition demonstrates a definite form or shape. However, the term cystic should not be used, because it implies that the lesion is not solid; a greater percentage of cases are solid.3 The interface with normal bone is known as the margin; it may be well defined, with or without reactive bone formation (“sclerotic halo”), or ill defined. In general, well-defined geographic lesions demonstrate less aggressive behavior and slower growth activity (Figure 20-2). Examples of geographic lesions include most benign bone tumors. If, over sequential studies, the margin becomes increasingly less defined, increased biologic activity should be suspected.4
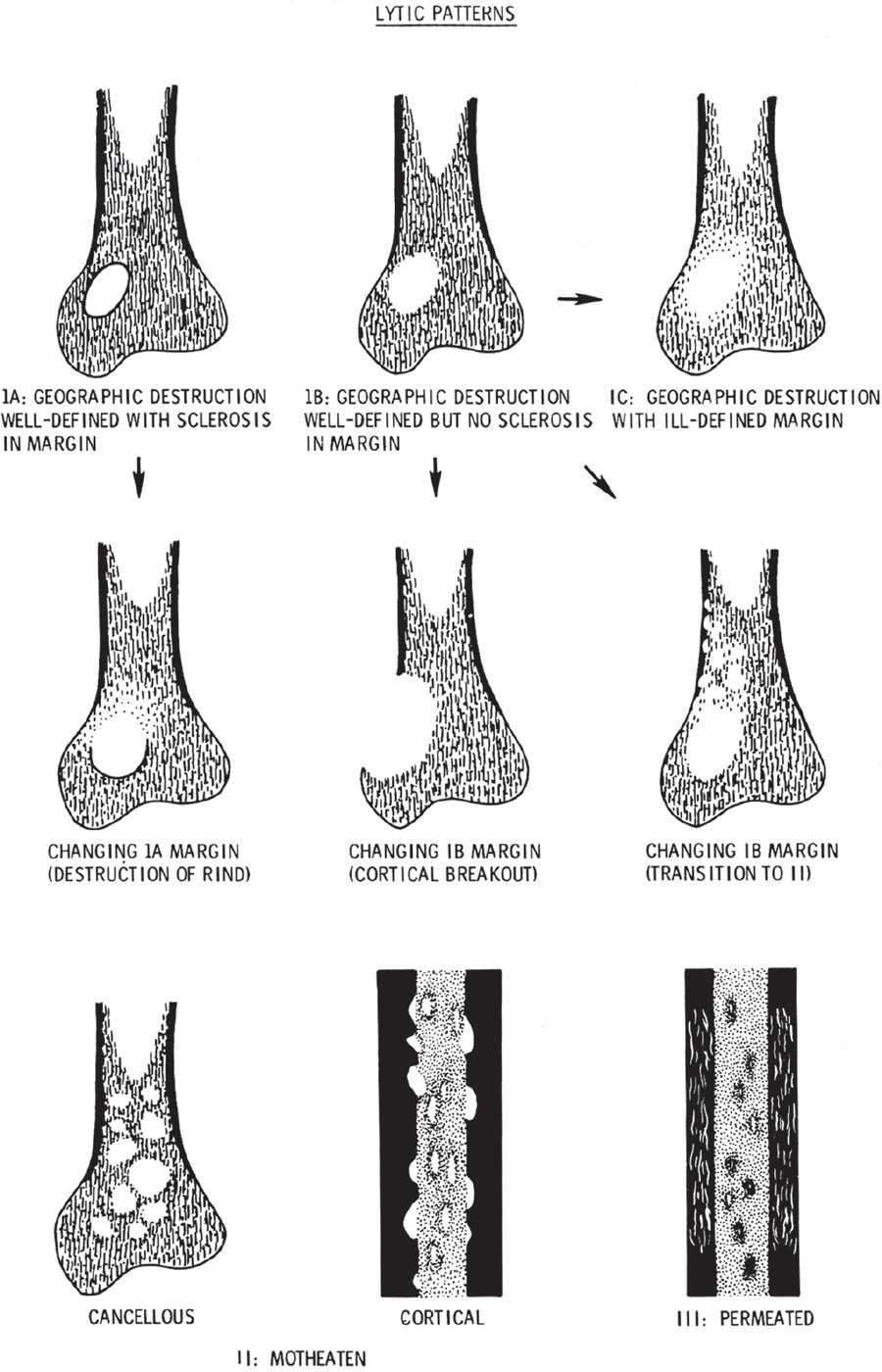
FIGURE 20-1. Schematic diagram of patterns of bone destruction and their margins. Arrows indicate the most frequent transitions or combinations of these margins. Transitions imply increased activity and a greater probability of malignancy. (From Madewell JE, Ragsdale BD, Sweet DE. Radiologic and pathologic analysis of solitary bone lesions: part I, internal margins. Radiol Clin North Am. 1981;19(4):722, figure 6.)
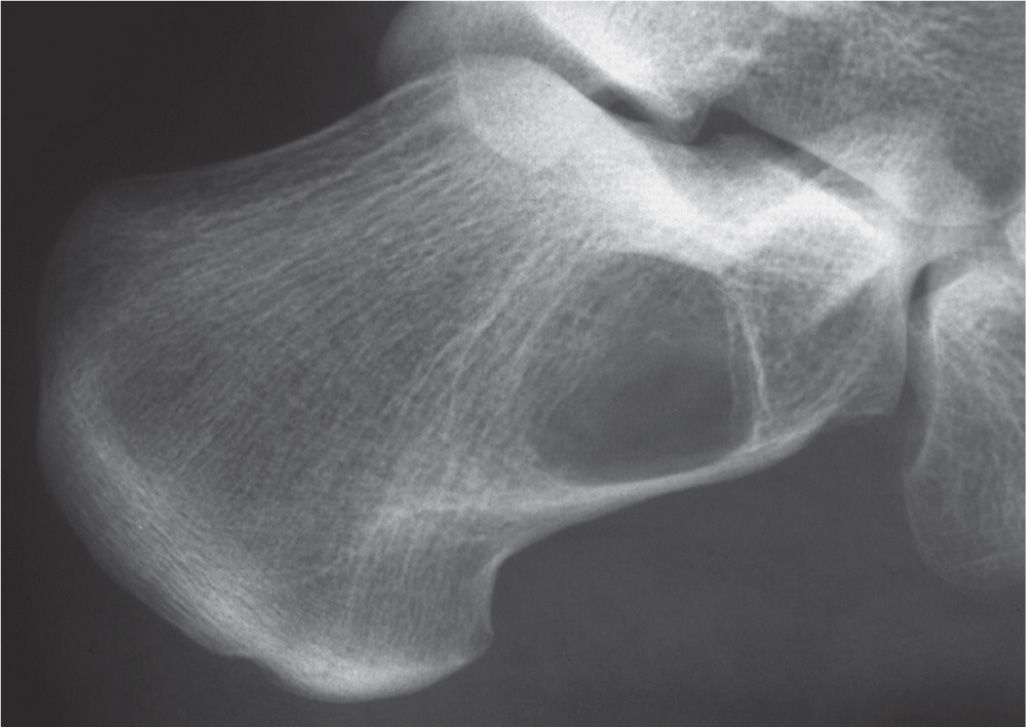
FIGURE 20-2. Calcaneal bone cyst demonstrating geographic grade IB destruction.
As noted earlier, well-defined geographic bone lesions bounded by a sclerotic margin tend to have slow growth activity (Figure 20-3). The sclerotic margin represents the surrounding normal bone’s attempt to wall off the lesion. In contrast, normal bone cannot be deposited quickly enough around an aggressive moth-eaten or permeative lesion to be visible radiographically. When a sclerotic margin rims a geographic lytic lesion, the lesion is usually slowly progressive and therefore probably benign in nature.5 It is nonetheless important to note that slow growth should not automatically be equated with the term “benign,” as low-grade malignant lesions (e.g., myxoid chondrosarcoma) may also manifest with well-defined geographic destruction.
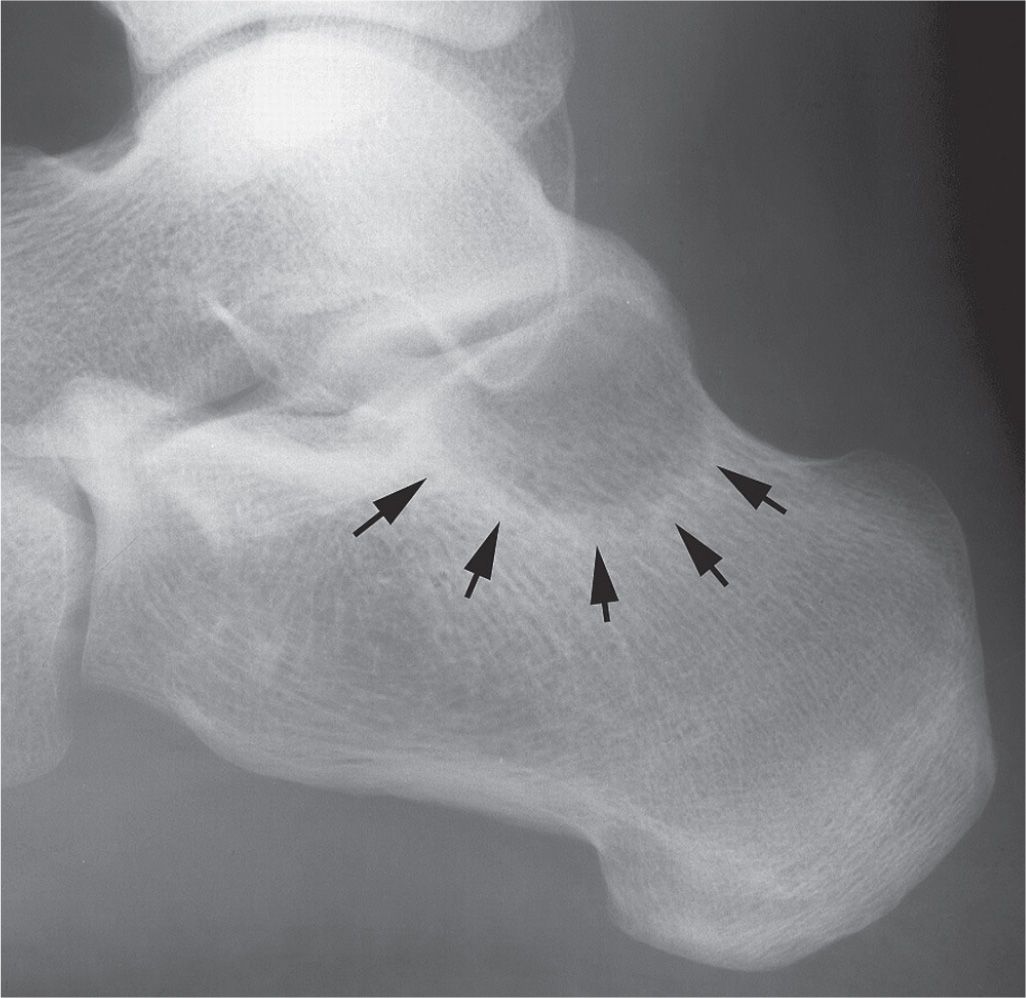
FIGURE 20-3. Calcaneal lesion demonstrating a geographic type IA destructive pattern; arrows indicate the sclerotic margin.
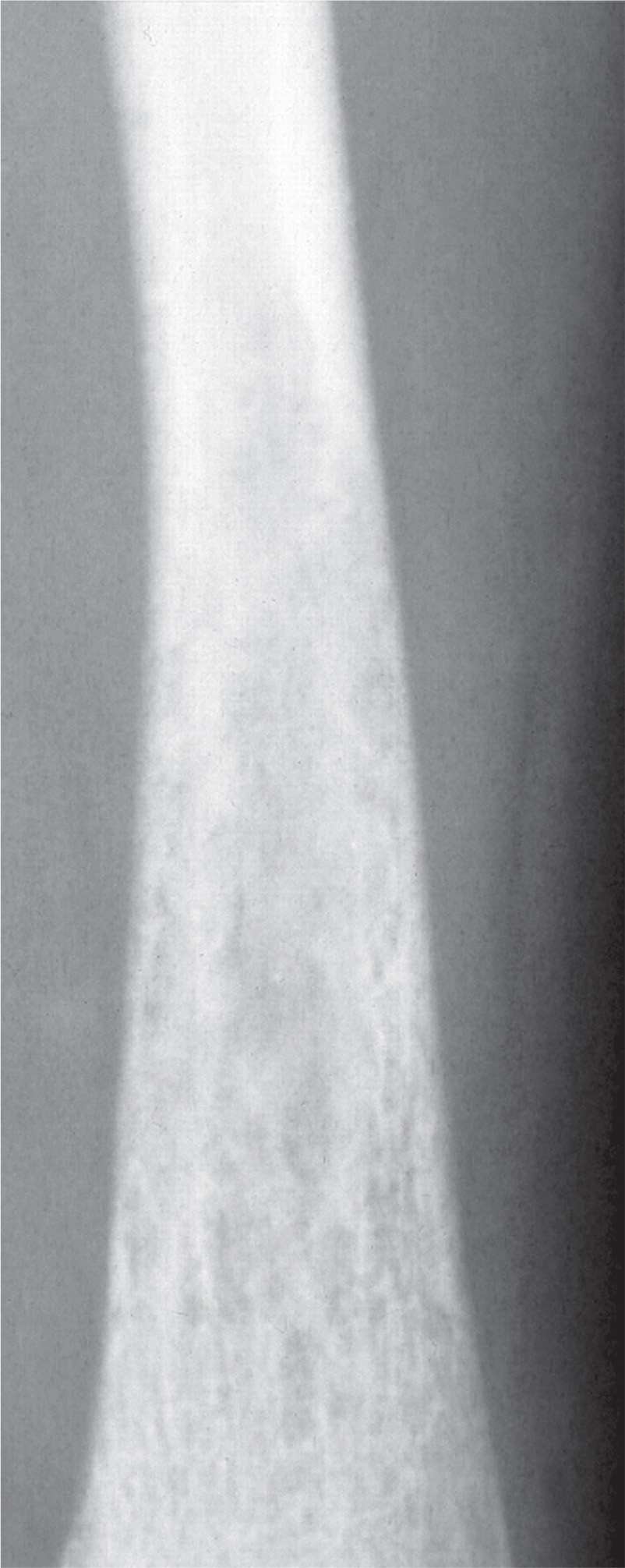
FIGURE 20-4. Permeated (type III) destruction involves the entire distal tibial diametaphysis, characteristic of fast growth rate.
When a narrow zone of transition exists between normal bone and lytic tumor, the lesion typically takes form, or has a defined shape. Therefore, in contradistinction, moth-eaten and permeative destructive patterns do not have a definite form or shape (Figure 20-4). Multiple, small, lytic lesions appear to spread across the affected area of bone. A moth-eaten pattern appears as multiple, small holes in cancellous or medullary bone; the permeative pattern typically presents as multiple, small streaks running through cortical bone. Lesions with either of these destructive patterns highly suggest aggressive activity. Examples include Ewing sarcoma, osteosarcoma, and untreated osteomyelitis. Acute osteopenia can also demonstrate a permeative or moth-eaten appearance, except it involves multiple bones (Figure 20-5).
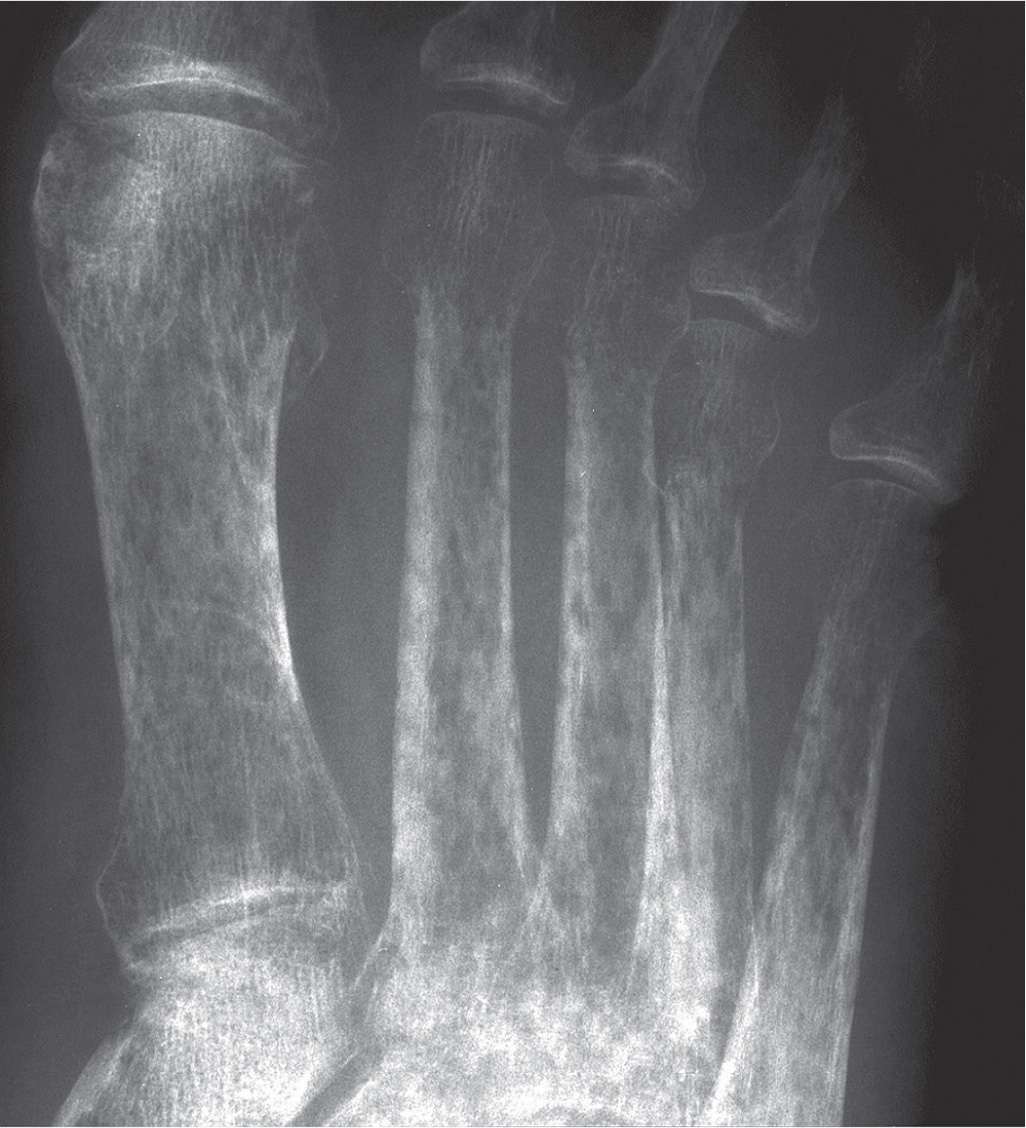
FIGURE 20-5. Rapidly forming, acute regional osteoporosis can demonstrate permeative (grade III) destruction of bone. In osteopenic disorders (e.g., complex regional pain syndrome), these patterns typically coalesce in the long term, leading to generalized osteopenia.
Size and Shape
Generally speaking, aggressive lesions tend to occupy larger areas of bone than slow-growing lesions. Regarding shape, geographic lytic lesions tend to be round to oval. As a general rule, lesions that appear in long bones during periods of skeletal growth and arise outside of the epiphysis tend to be oval, paralleling longitudinal bone growth, whereas intraepiphyseal lesions remain round. Evaluating the contour of the lesion can also be quite useful in foretelling the nature of a bone tumor or tumorlike condition. The contours of many lesions are smooth. However, when contours appear lobulated, cartilaginous and occasionally fibrous lesions are suspect. In some instances, outlines of geographic zones of pathology (not necessarily lytic lesions) are “serpiginous,” as typified by medullary infarcts or occasionally bone infection.
Cortical Involvement
A solitary bone lesion’s growth rate also can be estimated by the pattern of resorption along the adjacent cortex. Slow growth may only cause scalloping of the endosteal surface (Figure 20-6). With increasing growth rate, the cortex may be nearly fully resorbed yet still intact adjacent to the lesion. As endosteal resorption occurs, subperiosteal apposition may accompany it, giving the cortex an “expanded” appearance (Figure 20-7). A more aggressive lesion penetrates or breaks through the cortex and invades the soft tissues.
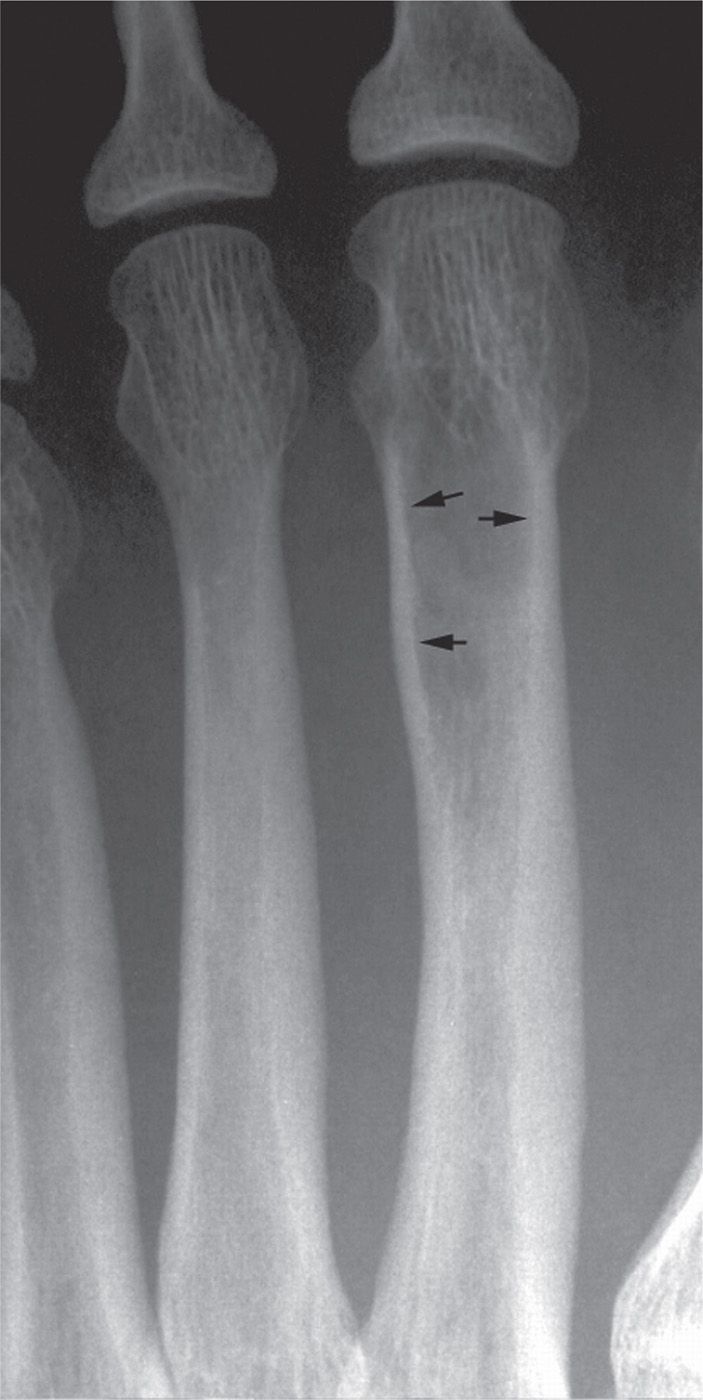
FIGURE 20-6. This lesion’s slow growth rate has resulted in scalloping of the endosteal surface of the cortex (arrows).
Periosteal Reaction
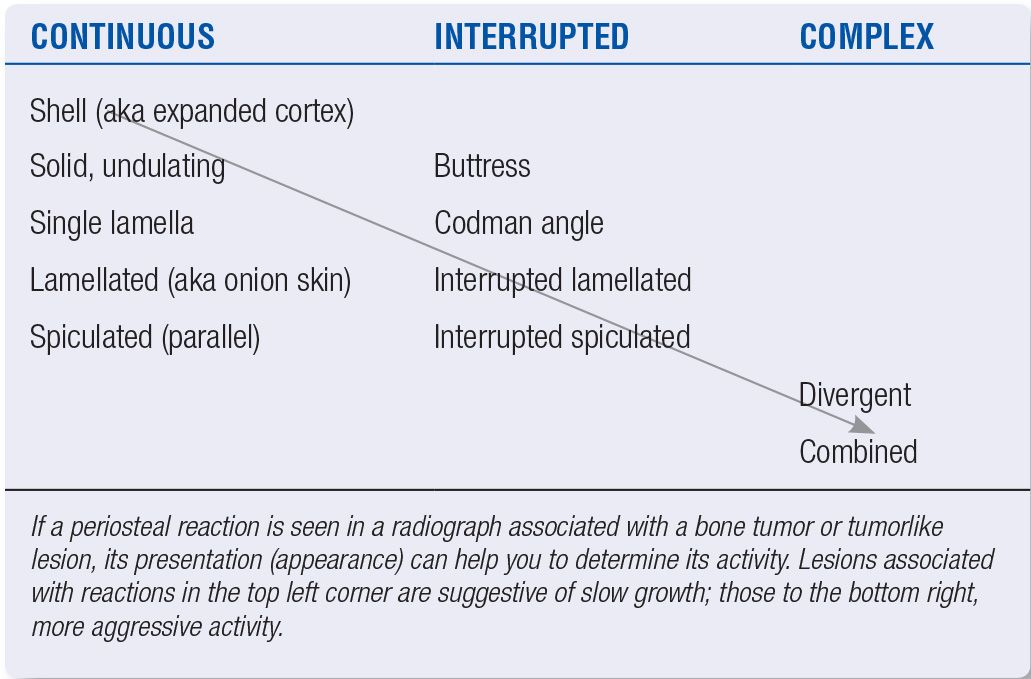
Periosteal reactions can have many different presentations (Figure 20-8). When a periosteal reaction is present, a degree of aggressiveness does exist, and the type of periosteal reaction present can be used to further estimate the growth rate (Table 20-2). For example, lesions that are accompanied by a continuous single layer or solid periosteal reaction are less aggressive than those demonstrating multiple layers, spicules, or fine lace-like patterns. Unless associated with a solid periosteal reaction, interrupted periosteal reactions suggest an aggressive lesion, as this signifies soft tissue extension and is therefore an upstaging sign. This is also the case with complex periosteal reactions.6 As a general rule, the more “porous” the periosteal pattern, the greater the likelihood of an aggressive lesion. Finally, layered appearances can be associated with underlying benign processes, especially when layers appear to be “stuck” or “glued” together (not distinctly separated by lucent layers).
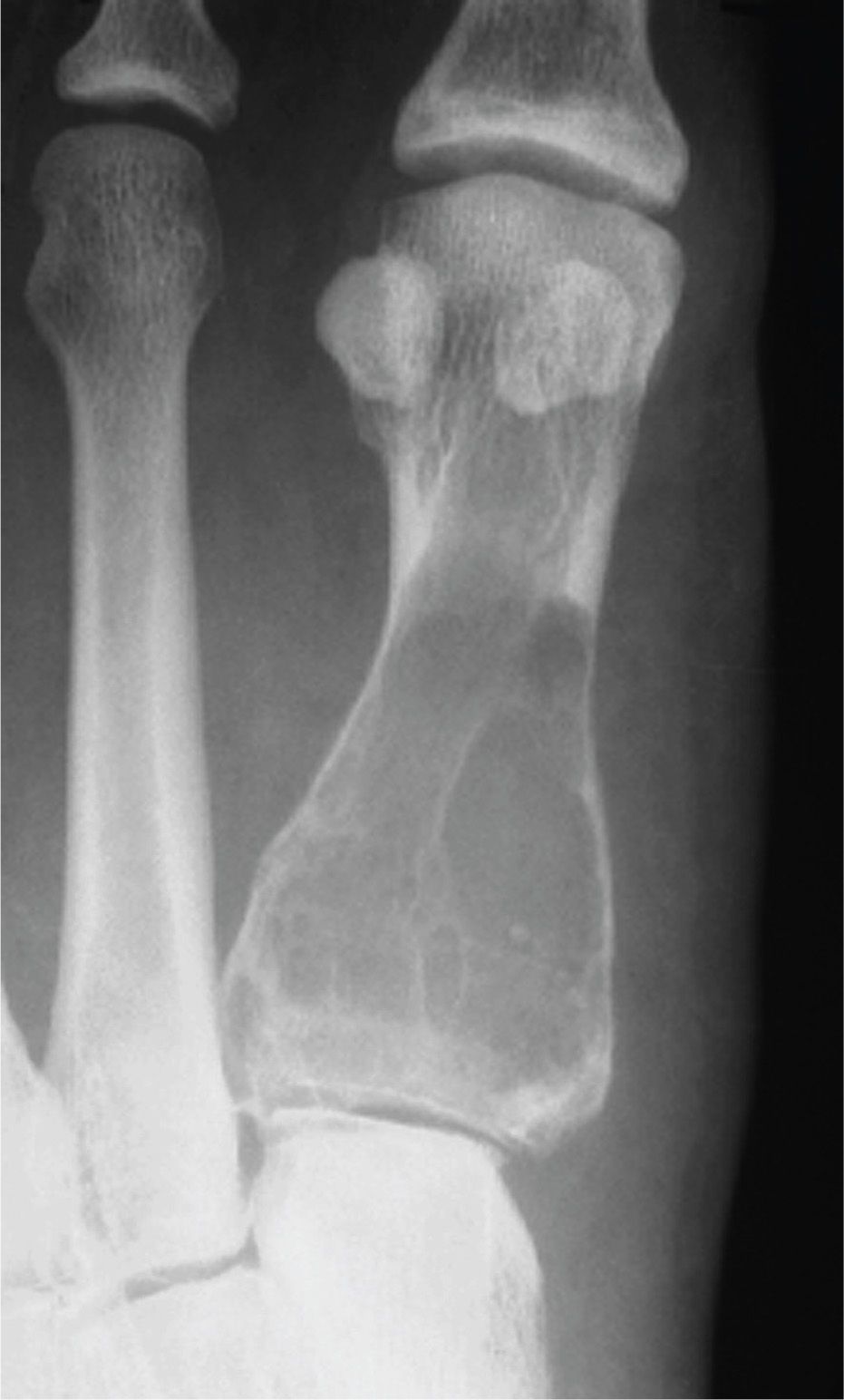
FIGURE 20-7. Chronic endosteal resorption caused by a slow-growing lesion and subsequent periosteal apposition results in the appearance of an “expanded” cortex.
Anatomic Position
The anatomic position of the lesion in a tubular bone can provide valuable diagnostic information. Many tumors tend to occur in specific anatomic locations. Conceptually, bone tumors are thought to spread like “waves on a pond,” and, in this regard, one should always endeavor to determine the center (or, epicenter) of a lesion. Position can be assessed in two planes of the image: horizontal and vertical (Figure 20-9). In the horizontal plane, the center of the lesion can have a position that is central (solitary bone cyst, fibrous dysplasia, enchondroma), eccentric (NOF, aneurysmal bone cyst [ABC]), cortical (osteoid osteoma), or parosteal (osteoma). A lesion’s vertical position can be epiphyseal (chondroblastoma, giant-cell tumor [GCT]), metaphyseal (enchondroma, chondromyxoid fibroma [CMF]), diametaphyseal (CMF, osteosarcoma), or diaphyseal (fibrous dysplasia, osteoblastoma, Ewing sarcoma).
Skeletal Location
Some solitary bone lesions have a predilection for certain bones. For example, enchondroma is frequently found in the phalanges of the hand and foot, although far more commonly situated in the hand. Osteoid osteoma may be found in the dorsal talar neck. Intraosseous lipoma and the solitary bone cyst are rare, but when encountered in the foot, they are most frequently situated in the calcaneal body.
Trabeculation
Trabeculation, when present, is found with only a few lesions (Box 20-1). Also, the type of trabeculation may further narrow the choice of diagnoses. Fine, delicate trabeculation (Figure 20-7) is associated with GCT; horizontally oriented fine, delicate trabeculation is a feature of ABC; coarse, thick trabeculations are seen with CMF; FCD has lobulated trabeculations that impart the appearance of “soap bubbles” (Figure 20-10); and radiating trabeculations are seen with hemangioma.7
Matrix Production
Most tumors do not radiographically demonstrate visible extracellular matrix. However, for those that do, matrix mineralization can narrow the list of differential diagnoses for any particular lesion to a specific category of primary bone tumors (Figure 20-11).8 Ossific mineralized matrix patterns manifest with solid, cloud-like, homogeneous or “ivory-like” increased density. Examples of osteoblastic tumors include osteoblastoma and osteosarcoma. In contradistinction, cartilaginous or chondroid matrix patterns typically present with stippled, punctate, flocculent, popcorn-like, or curvilinear areas of increased density. Although these patterns are mainly encountered in cartilaginous tumors, they may also be noted in areas of tissue necrosis and infarction. Examples include chondroma (Figure 20-12) and chondrosarcoma. Occasionally, a periphery of “pearls” of neoplastic cartilage will calcify yielding a “rings-and-arcs” appearance. Fibroblastic lesions generally do not radiographically demonstrate a matrix and therefore appear lucent, with exception of fibrous dysplasia, which can produce the so-called “ground-glass” matrix, which appears smoky, milky, or smudgy.
Clinical Course
The clinical presentation of most tumors is nonspecific; complaints may include pain and swelling. Inflammatory signs are not necessarily present. In some instances, pain is secondary to pathologic fracture. There are a few instances where lesions have a fairly characteristic clinical presentation. One well-known example is osteoid osteoma, which classically presents with night pain that is relieved by aspirin.
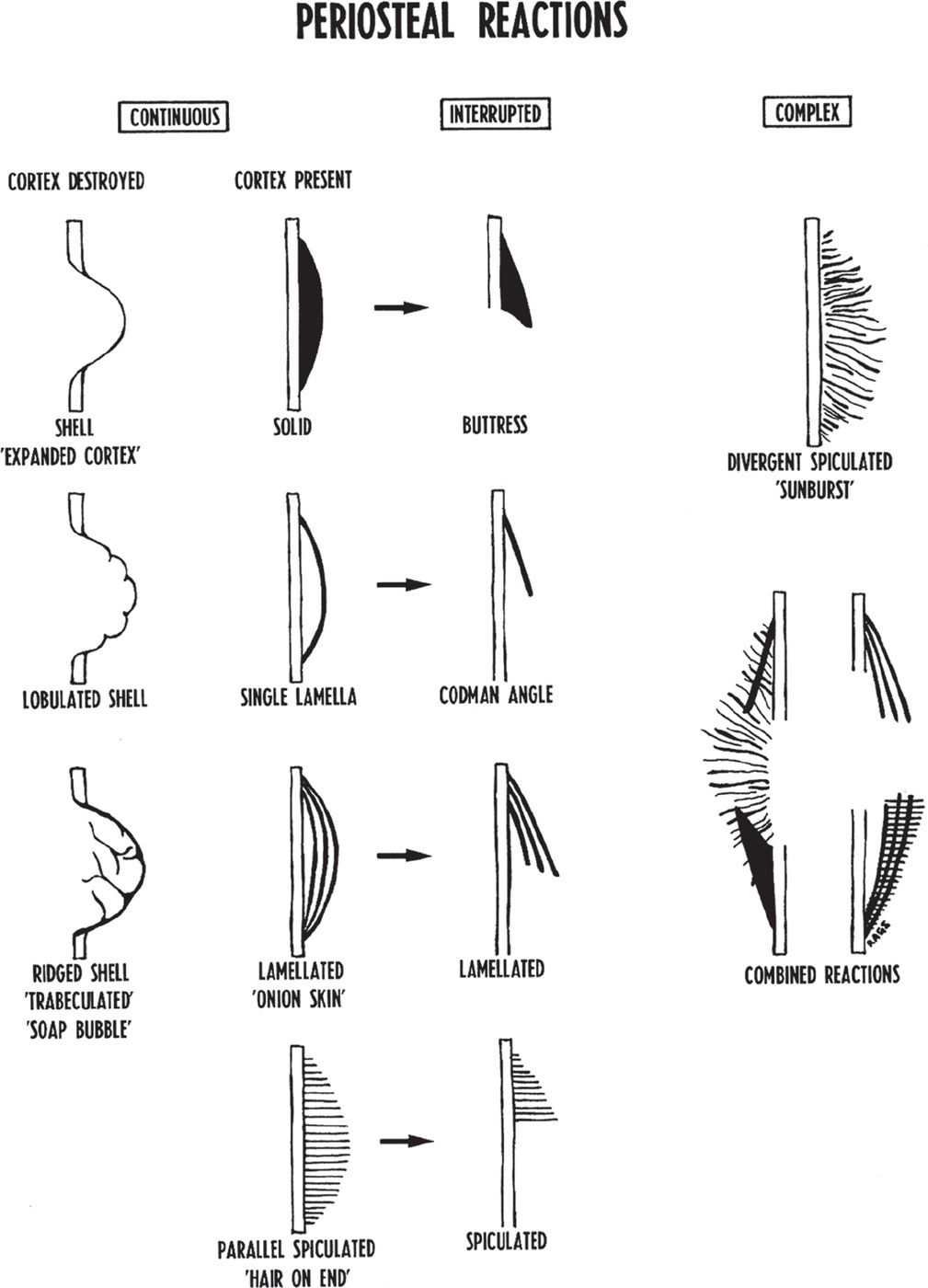
FIGURE 20-8. Schematic diagram of periosteal reactions. The arrows indicate that the continuous reactions may be interrupted. (From Ragsdale BD, Madewell JE, Sweet DE. Radiologic and pathologic analysis of solitary bone lesions: part II, periosteal reactions. Radiol Clin North Am. 1981;19(4):751, figure 2.)
| Periosteal Reactions and Lesion Activity |
Age of Patient
Most bone tumors tend to have peak occurrences in certain age ranges (Table 20-3).9 For example, Ewing tumor, simple bone cyst, and chondroblastoma are found in the first two decades of life. In contrast, multiple myeloma, fibrosarcoma, and metastasis present in the fifth to seventh decade.
Adjunctive Imaging Studies
The use of ancillary imaging modalities such as CT and MRI in the evaluation of musculoskeletal lesions is routine nowadays, and it has also become commonplace in the approach to bone tumors and tumorlike lesions. Some of the significant advantages over radiography that both MRI and CT inherently possess include sectional imaging, improved resolution (especially contrast resolution with MRI for soft tissue evaluation), ability to detect fluid–fluid levels, and ability to employ contrast agents like gadolinium (for MRI) to help characterize lesions and accurately determine extent. Nevertheless, CT, MRI, nuclear medicine, positron emission tomography (18FDG-PET), and ultrasound are rightfully labeled “ancillary” imaging modalities, as radiography is still the imaging modality of first choice.
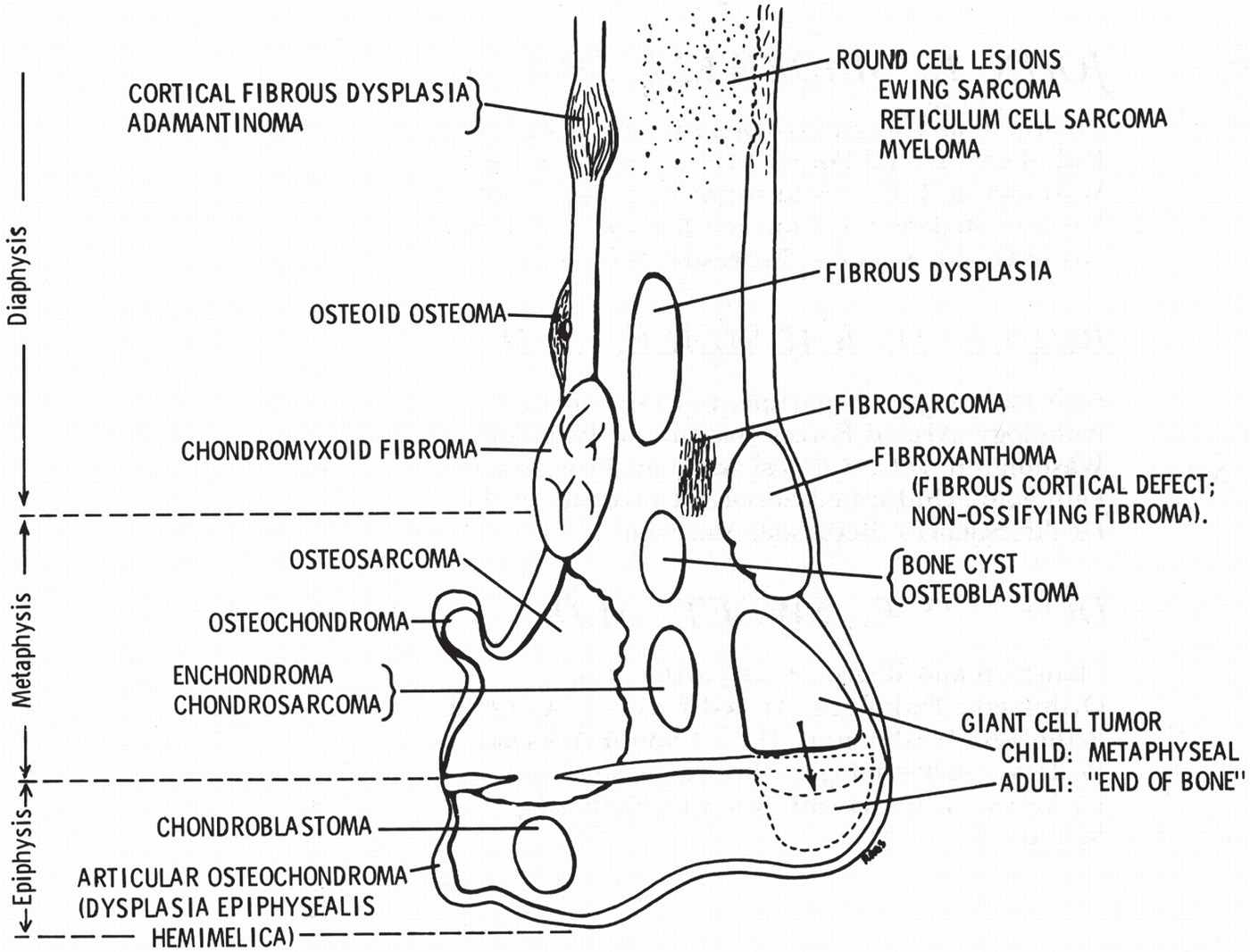
FIGURE 20-9. Composite diagram illustrating frequent sites of bone tumors. The diagram depicts the end of a long bone that has been divided into the epiphysis, metaphysis, and diaphysis. The typical sites of common primary bone tumors are labeled. Bone tumors tend to predominate in those ends of long bones that undergo the greatest growth and remodeling; hence, they have the greatest number of cells and amount of cell activity (shoulder and knee regions). When small tumors, presumably detected early, are analyzed, preferential sites of tumor origin become apparent within each bone (as shown in this illustration), suggesting a relationship between the type of tumor and the anatomic site affected. In general, a tumor of a given cell type arises in the field where the homologous normal cells are most active. These regional variations suggest that the composition of the tumor is affected or may be determined by the metabolic field in which it arises. (From Madewell JE, Ragsdale BD, Sweet DE. Radiologic and pathologic analysis of solitary bone lesions: part I, internal margins. Radiol Clin North Am. 1981;19(4):716, figure 1.)
BOX 20-1 Tumors Demonstrating Trabeculation
Giant-cell tumor
Aneurysmal bone cyst
Chondromyxoid fibroma
Hemangioma
Fibrous cortical defect
SELECTED MALIGNANT TUMORS
The “big three” primary mesenchymal malignancies—osteosarcoma, chondrosarcoma, and fibrosarcoma—are rarely encountered in the foot.
Osteogenic Sarcoma (Osteosarcoma)
Osteogenic sarcoma is an aggressive bone tumor pathologically classified as a malignant mesenchymal neoplasm. The tumor cells directly produce immature bone, which is characterized by profound osteoid elaboration by sarcomatous cells. Pathologically, osteoid may be mixed with cartilage and/or fibrous tissue of varying amounts, and this may have radiologic consequences. Statistically, osteogenic sarcoma is the second most common primary malignant bone tumor bodywide, second only to multiple myeloma, and is approximately three times as common as Ewing sarcoma. That being said, it should be noted that when the foot is involved, several larger series have found Ewing sarcoma to be more prevalent.10,11 There are a number of osteosarcoma subtypes, and even extraskeletal variants are known (Table 20-4). Nevertheless, the majority (approximately 75%) of all lesions are intramedullary osteosarcomas. In addition to primary lesions, osteosarcomatous degeneration has been described in association with a number of benign conditions such as Pagetic bone, chronic osteomyelitis, osteonecrosis, fibrous dysplasia, and osteochondroma.12
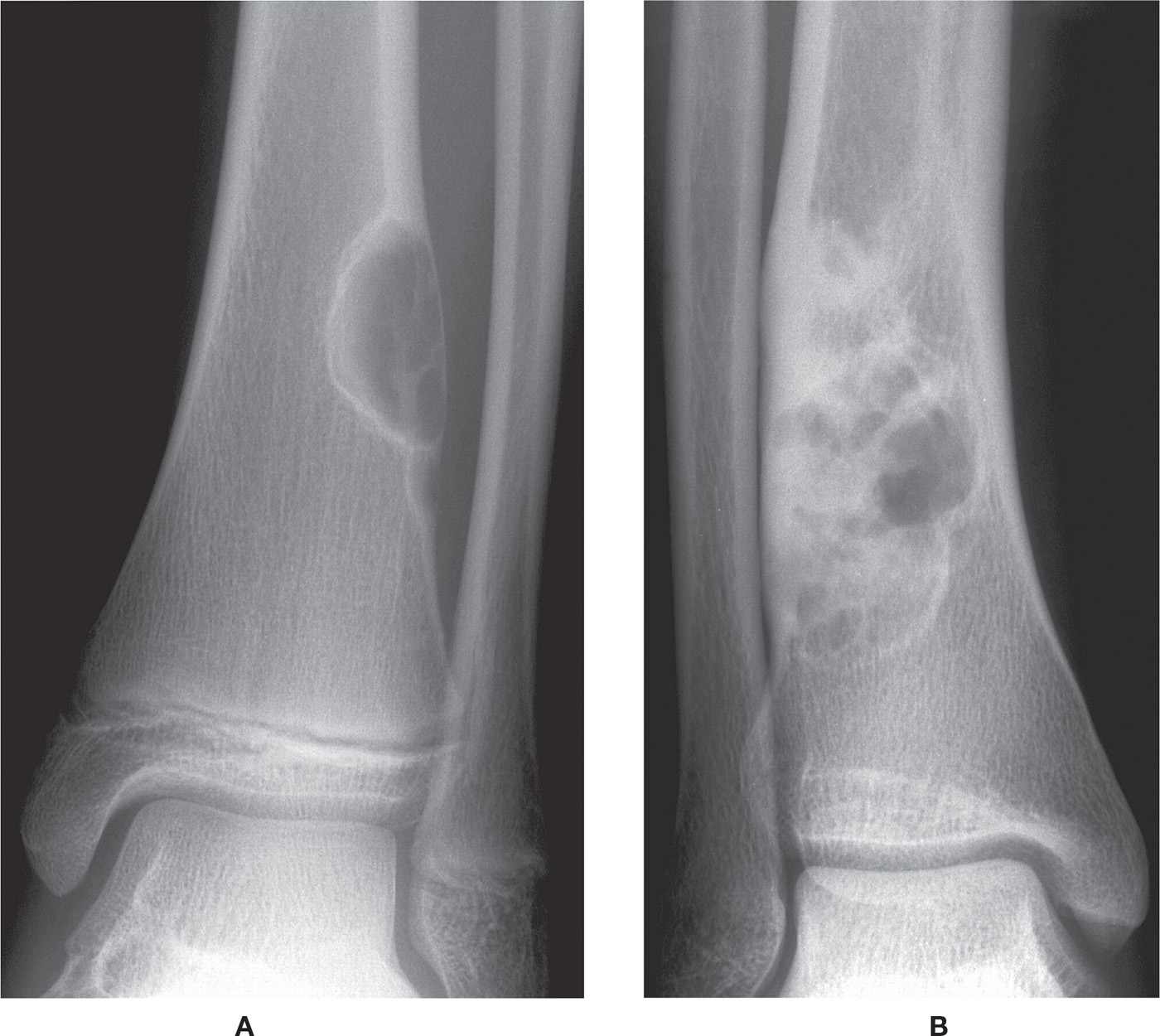
FIGURE 20-10. Examples of fibrocortical defects (NOFs) at varying stages of development. A: The trabeculations appear fine and delicate in early stages. B: In an older patient, the lesion remodels, and the “soap-bubble” appearance is more obvious.
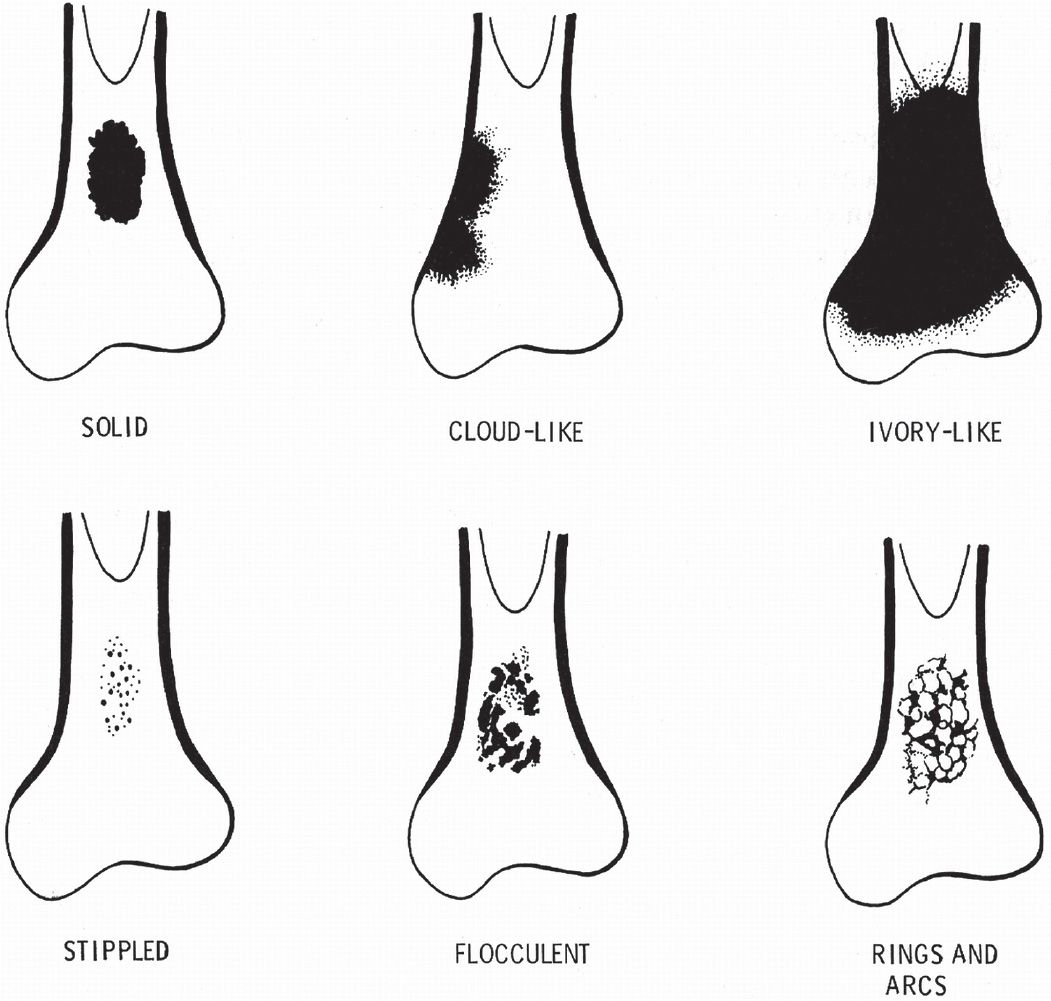
FIGURE 20-11. Schematic diagram of mineralized matrix patterns. Tumor osteoid appears as increased density with a solid (sharp-edged) or cloudy to ivory-like (ill-defined edge) pattern. Tumor cartilage creates stippled, flocculent, and solid density patterns. Rings and arcs represent bony rims around tumor cartilage lobules. Dystrophic mineralization and ischemic osteoid tend to mimic the stippled, flocculent, or patchy solid density pattern. (From Sweet DE, Madewell JE, Ragsdale BD. Radiologic and pathologic analysis of solitary bone lesions: part III, matrix patterns. Radiol Clin North Am. 1981;19(4):788, figure 1.)
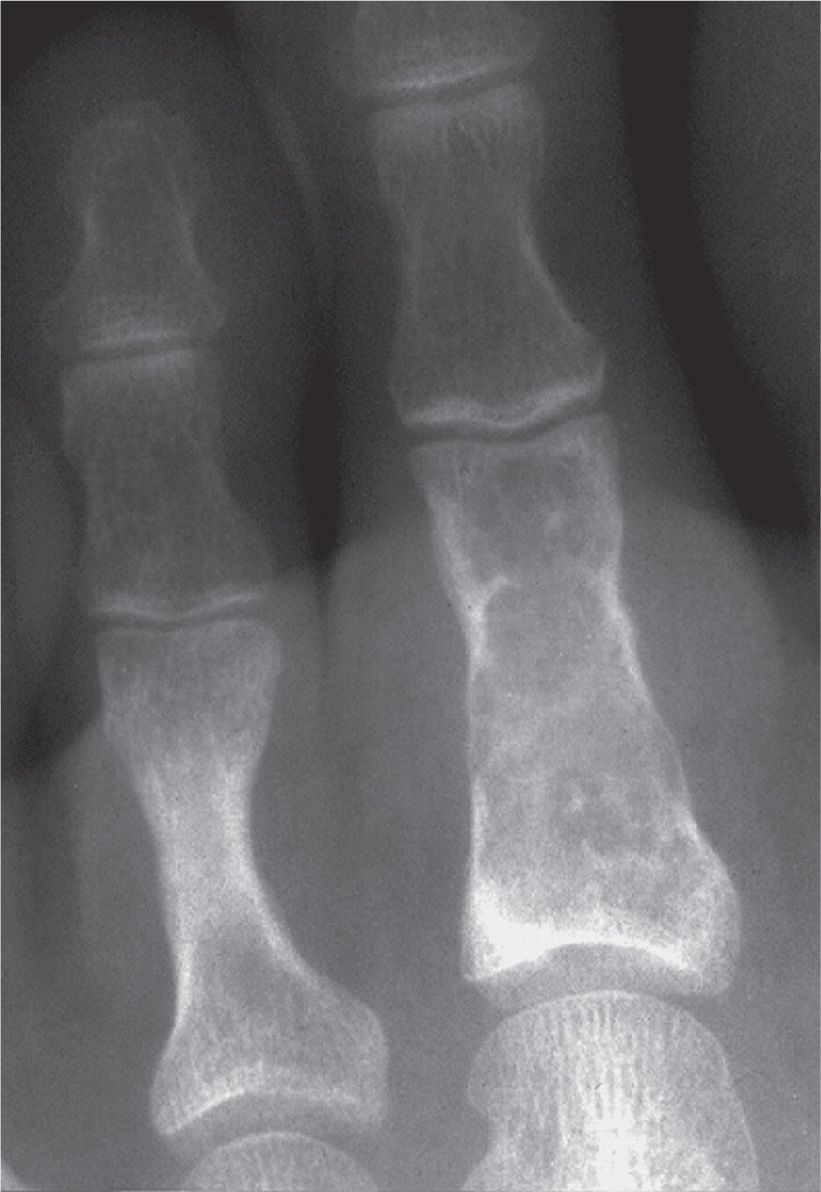
FIGURE 20-12. Enchondroma, second toe proximal phalanx, demonstrating a rings-and-arcs mineralized matrix pattern. (Courtesy of Gary F. Bjarnason, Roanoke Rapids, NC.)
| Age Ranges of Bone Tumor Distributiona |
Tumor | Peak Age Range (Years) |
Benign Tumors | |
Osteoma | 15–45 |
Osteoid osteoma | 10–23 |
Benign osteoblastoma | 10–30 |
Osteochondroma | 10–30 |
Central chondroma | 10–40 |
Chondroblastoma | 10–20 |
Chondromyxoid fibroma | 10–30 |
Eosinophilic granuloma | 5–10 |
Nonosteogenic fibroma | 10–20 |
Desmoplastic fibroma | 10–30 |
Intraosseous lipoma | 30–50 |
Neurilemoma | 10–30 |
Hemangioma | 40–50 |
Giant-cell tumor | 25–40 |
Simple bone cyst | 5–20 |
Aneurysmal bone cyst | 10–30 |
Enchondroma | 30s |
Primary Malignant Tumors | |
Osteogenic sarcoma | 10–20 |
Parosteal osteosarcoma | 20–40 |
Chondrosarcoma | 30–60 |
Fibrosarcoma | 30–40 |
Malignant giant-cell tumor | 30–50 |
Adamantinoma | 10–30 |
Hemangioendothelioma | 30–40 |
Ewing sarcoma | 10–20 |
Reticulum cell sarcoma | 30–60 |
Myeloma | 50–80 |
Chordoma | 30–60 |
Other Tumors | |
Leukemia: Acute | 2–6 |
Leukemia: Chronic | 40–70 |
Metastatic neuroblastoma | <4 |
Metastatic carcinoma | 40–80 |
aModified from Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8542 Cases. 4th ed. Springfield, IL: Charles C Thomas; 1986.
| Basic Classification of Osteogenic Sarcoma |
Intraosseous
Intramedullary high grade (aka conventional)
Telangiectatic
Small cell
Low grade
Gnathic
Surface
Periosteal
High-grade periosteal
Juxtacortical (parosteal)
Intracortical
Extraosseous
Secondary
Multicentric
Epidemiology
As noted earlier, osteosarcoma is considered to be the second most common primary bone tumor, and is generally the most common primary malignant bone tumor of adolescents and young adults. Despite this, only about 1% of all osteosarcomas are found in the foot, making them decidedly uncommon. Males are involved about twice as often as females. Age data show peak incidence in adolescence, with a median of about 17 years for tubular bones and 25 years for flat bones. A second, older age peak is encountered for those secondary osteosarcomas that arise in pathologic bone largely associated with Paget disease.
Location
The knee is involved in approximately 50% of cases. In order of decreasing occurrence, the most common sites are the distal femur, followed by the proximal tibia and proximal humerus. The origin is usually metaphyseal, although, in 10% to 20% of cases, the lesion may be centered in the diaphysis. Epiphyseal origins are encountered in less than 1%.13 Nevertheless, in long bones, extension of the tumor into any of these sites may be seen. In light of the decreased size of pedal bones, by the time of discovery, lesions frequently involve the entire bone. In one large study, only 2.9% (101 of 3482) of cases involved the foot and ankle, primarily due to ankle (distal leg) involvement. Consistent with other studies of these lesions involving the foot and ankle, less than 1% (27 of 3482) involved the foot,14–21 and most of the lesions were of the conventional/high-grade intramedullary variety. Although the rate of occurrence in the foot is infrequent, osteogenic sarcoma is still considered to be the most common primary malignant tumor in this region, especially in childhood.21
Metastasis
Metastasis is most common to the lungs; occasionally, there is osteosarcomatous metastasis to the viscera or other parts of the skeletal system. Rarely metastasis occurs via the lymphatics, with regional lymph node involvement.
Pedal Location
When the foot is involved, the majority of osteosarcomas (about 70%) are located within the tarsal bones, with the os calcis the most frequent site. The metatarsal bones are the second most common location, with metaphyseal or diaphyseal involvement. Conventional osteosarcoma, as well as a number of subtypes, has been reported in the metatarsals.22–27 Osteosarcoma of the phalanx is rare, but has been reported.27–33 In the series by Anninga et al.,27 the distribution of foot bones was tarsal bones (56%), metatarsal bones (33%), and phalanges (11%).
Radiographic Features
Osteosarcoma presents as a bony lesion that is either densely sclerotic (40%–45%), osteolytic (25%), or mixed lytic–sclerotic (25% to 30%). Therefore, signs of an ossific matrix are present in 75% of cases. Uniformly dense lesions are typically encountered in late stages.34–37 Inasmuch as the bony cortex is freely penetrated or permeated, periosteal reactions are typically present, although lesions rarely appear expansile. Intramedullary lesions are thought to arise in the metaphysis and are commonly centered in the metaphysis or diametaphysis of long bones. However, it is important to note that diaphyseal involvement is fairly common. Whereas articular cartilage generally presents a barrier to penetration of the joint, direct penetration through a physis is a notable feature of osteogenic sarcoma.38
The ossific matrix results in densely sclerotic or mixed lytic–sclerotic changes to bone in most cases. The more homogeneous the radiographic density or advanced the state, the greater the difficulty in distinguishing new bone formation from a reactive sclerotic response of normal bone in other chronic conditions. In long bones, Ewing sarcoma does not typically demonstrate a visible matrix and is largely epicentered in the diaphysis, allowing for relatively easy delineation from osteosarcoma. Unfortunately, reports of Ewing sarcoma in the tarsal bones indicate that reactive medullary sclerosis is typical, and, therefore, radiographic distinction from osteosarcoma can be problematic, if not impossible, in the absence of soft tissue ossification.
Aggressive periosteal reaction patterns are classic, in particular, the complex, spiculated “sunburst” type. “Hair-on-end” periosteal spiculation is also seen as well as multilaminated reactions. The Codman angle periosteal reaction is typical at sites of extraosseous extension.
Internal lysis is manifested by type III moth-eaten destruction; less commonly permeative (type II) destruction may be apparent. Again, this is often obscured on the radiograph as neogenic ossification progressively fills these lytic voids. Geographic lysis with IC-type margination has occasionally been observed. Cortical erosion is variable, but not rare.
Soft tissue invasion is typical, radiographically suggested by the presence of a Codman angle periosteal reaction. In one study by Hudson et al.,39 soft tissue masses were visible in 41 of 47 cases. In about 50% of these cases, dense, cloudy bone was contained within the soft tissue mass.
Osteosarcoma is notorious for its ability to extend across an open physis. Pathologic studies demonstrate that from 75% to 88% of metaphyseal osteosarcomas demonstrate epiphyseal extension through the physis.12,40,41
Chondrosarcoma
With a number of variant/subgroups described, chondrosarcoma now more appropriately refers to a group of malignant tumors that are predominantly cartilage. They demonstrate a range of behavior from low-grade activity and low metastatic potential to high-grade tumors with early metastasis. Based on location, three general types are now recognized: central, peripheral, and juxtacortical/periosteal. Most are central in nature, within the medullary canal (approximately 85%); the peripheral variety arises in and around the cortex and occurs in approximately 15% of lesions. Those that occur at the juxtacortical/periosteal surface of bone (periosteal chondrosarcoma) comprise less than 1%.12,13 Additionally, chondrosarcoma is classified as either primary or secondary.
Primary chondrosarcoma is more common than the secondary form. It arises as a de novo malignant lesion, generally in middle-to-older age patients. Many subtypes of primary chondrosarcoma have been identified. These include juxtacortical, clear-cell, myxoid, mesenchymal, extraskeletal, and dedifferentiated chondrosarcoma.
Secondary chondrosarcoma is malignant degeneration of a preexisting benign lesion, often cartilaginous (e.g., osteocartilaginous exostosis, hereditary multiple exostoses, enchondroma, or in Pagetic bone). It occurs more commonly in younger patients.
In general, proximal chondrosarcoma tends to be primary, whereas the peripheral chondrosarcomatous lesion tends to arise from degeneration of enchondroma (most common), osteochondroma (occasional), or Paget disease (rare).
Grading
Chondrosarcoma is generally classified into three histologic grades, depending on the degree of cellularity, cellular atypia, and pleomorphism.42 The higher the grade, the greater likelihood of metastasis. Grade I lesions rarely metastasize, whereas approximately 10% to 15% of grade II lesions and greater than 50% of grade III lesions metastasize.
Clinical Features and Epidemiology
Chondrosarcoma is the third most common primary malignancy of bone, behind multiple myeloma and osteosarcoma. The male-to-female ratio is 1.5:1.43 Chondrosarcoma represents about 10% of all primary bone tumors; they occur about half as often as osteosarcoma. As noted earlier, there is a decided tendency for older age involvement; 50% of central chondrosarcomas occur in patients over the age of 40. The mean age for peripheral (secondary) chondrosarcoma is considerably lower, that is 33 years.44–46 Chondrosarcoma is rarely found in children. Hand and foot involvement is unusual (about 3%–5% of all lesions).47–51 Nonetheless, it is considered the most common primary malignant bone tumor of the hand or foot in the middle-aged patient. When one factors in secondary degeneration of enchondroma, hand involvement (not surprisingly) is greater than the foot involvemnt. Nonspecific, dull pain (occasionally nocturnal) and gradual swelling are the most common presenting symptoms of chondrosarcoma, without antecedent trauma. Tumor growth is usually slow (unless high grade) with late bloodstream metastasis. However, childhood chondrosarcoma is generally more aggressive than the adult forms. Pathologic fracture is uncommon and is usually seen in higher-grade tumors.
Location
Chondrosarcoma may be found in any bone with cartilaginous origin. Bodywide, the extremity long bones, pelvis, scapula, and ribs are the most common sites. In the foot, the calcaneus appears to be most frequently involved, followed by the talus.51–54 However, chondrosarcoma of the foot has also been noted in the lesser tarsal bones, phalanges, and metatarsals. Recently, Kwon et al.55 reported a case of skeletal myxoid chondrosarcoma in a 20-year-old male in the neutral triangle region.
Most chondrosarcomas in tubular bones are the central type, and commonly involve the metaphysis and metadiaphyseal areas (mesenchymal cell lesions). Epiphyseal and/or apophyseal involvement is rare.
General Radiographic Features
Unless arising from a preexisting lesion, such as a sessile osteochondroma, or if a calcific matrix pattern exists, the picture can frequently be nondescript. With respect to lysis, it is important to realize that there is generally a close correlation with malignancy grade and aggressiveness of radiographic features.56 Soft tissue mass, deep endosteal scalloping, cortical break through, periosteal reaction, and large size tend to suggest malignancy. On the other hand, low-grade chondrosarcoma may demonstrate geographic destruction and well-defined (albeit scalloped) margins. Unfortunately, the majority of conventional central chondrosarcomas are slow-growing, low-grade lesions that do not present with aggressive radiographic features. For example, the radiographic picture of myxoid chondrosarcoma commonly presents with benign features.55
Radiographic Features of Conventional Intramedullary Chondrosarcoma
It appears as a geographic lytic lesion of bone with poorly defined margins (type IC), but occasionally demonstrates type II or III destruction; the latter are more commonly encountered with mesenchymal, myxoid, and dedifferentiated histologic subtypes.
Scalloped inner cortical (endosteal) erosion is seen, which is consistent with lobular growth. In long bones with thick cortices, scallops tend to be deeper (greater than two-thirds of the cortical width) for chondrosarcoma as opposed to enchondroma.51,56,57
Although thinning of the cortex may be seen, cortical thickening is a distinctive imaging feature. In concert with this, periosteal reactions are variable and appear to correlate with the degree of cortical penetration. As noted before, solid periosteal reactions generally indicate benign processes. However, the well-known exception to this rule is that chondrosarcoma of long bones may demonstrate solid or lamellated periosteal new bone formation.58
Evidence of calcified matrix (punctate/stippled, “fluffy,” flocculent, or curvilinear “ring-and-arc” formations) is demonstrated in about 65% of lesions. In some cases, calcification can be so dense so as to be confused with a more homogeneous ossific matrix of osteosarcoma or reactive sclerosis of Ewing tumor (especially the os calcis).
Soft tissue mass occasionally presents with or without calcification. Pathologic fracture occasionally occurs, generally with extensive cortical remodeling.
The clear-cell pathologic variant of chondrosarcoma is known for epiphyseal and epimetaphyseal involvement, geographic destruction with well-defined sclerotic margin (type IA), negative cortical destruction or periosteal new bone formation, and variable calcification. This variant is notorious for radiographically simulating other benign epiphyseal lesions such as chondroblastoma.
Basic Differential Diagnosis
The primary differential diagnosis is benign enchondroma. In the foot, Gajewski et al.59 determined that size and location were significant discriminators with respect to secondary chondrosarcoma. Specifically, enchondromas had a smaller mean size of 2.7 cm, and those lesions that occurred in the hindfoot and midfoot were more likely to be malignant than those in the forefoot. In addition, benign cartilage lesions can be radiographically difficult to differentiate from slow-growing, low-grade chondrosarcoma.
Ewing Sarcoma
One of the most lethal primary bone tumors, this lesion was initially described by Ewing in 1921.17,60–68 Ewing sarcoma is now considered part of a group of tumors, probably of neuroectodermal origin, that share the same cytogenetic marker. These include Ewing sarcoma of bone, extraskeletal Ewing, primitive neuroectodermal tumor, and Askin tumor (extraskeletal Ewing sarcoma of the thoracopulmonary region). Specifically, all of these lesions share in common the nonrandom reciprocal translocation between chromosomes 11 and 22.69 Along with myeloma and non-Hodgkin lymphoma (reticulum cell sarcoma), Ewing sarcoma is classified as a non matrix-producing round-cell tumor. Histologically (with H & E stain), these lesions demonstrate crowded sheets of “monotonous” small round blue cells.
Epidemiology/Prevalance
After osteosarcoma, skeletal Ewing sarcoma is considered to be the second most common primary malignant tumor of bone in young children and adolescents. Overall, it is ranked as the fourth most frequent primary malignant tumor of bone behind multiple myeloma, osteosarcoma, and chondrosarcoma. Despite this, pedal Ewing sarcoma is quite rare with about 0.5% arising in the foot.
Location
Almost any bone in the body can be involved. It has a decided predilection for long bones, greater in the lower extremity. In the foot, most cases appear to be split between the calcaneus and metatarsals. There have, however, been case reports in the toes and other tarsal bones. In the os calcis, which is the most frequent pedal site for Ewing sarcoma, the lesion often tends to have its epicenter at the junction of the posterior body and greater tuberosity (where vestigial red marrow may occur in some adults).
Clinical Features
Pain may become severe and recalcitrant. Pathologic fracture is often the initial presentation. With extension of the neoplasm into the soft tissues, swelling becomes apparent. Ewing sarcoma is predominantly a young person’s tumor, with predilection for Caucasians. The peak age incidence is 15 years, with a range between 5 and 30 years. Under age 5, metastatic neuroblastoma should be suspected. Over age 30, one should suspect reticulum cell sarcoma. The male-to-female ratio is 3:2.
Radiographic Features
The classic radiographic features of Ewing sarcoma in long bones are mirrored in the small bones of the hands and feet. They include lytic, permeative destruction, aggressive periosteal reaction, cortical violation and breakthrough, and soft tissue mass. These findings are more likely to be encountered in the metatarsal and phalangeal bones; tarsal bones will demonstrate atypical features.
Ewing sarcoma classically presents in the diaphyseal to diametaphyseal region of long bones. Poorly defined permeative to moth-eaten destruction (type III growth rate) is noted in the majority of lesions (approximately 75%–80%). Inherently associated with a wide zone of transition, this reflects the highly aggressive nature of Ewing sarcoma, and ancillary imaging such as MRI and CT is far more reliable in determining the extent of the lesion. In the smaller tubular bones, although lesions appear to originate in the metadiaphyseal or diaphyseal regions, extension to the end of bone may be seen. In the study by Baraga et al.,70 19% (of 43 hand and foot cases) demonstrated total involvement of a short tubular bone. Rarely, sharply defined or even cystic lesions have been reported, simulating more benign lesions like ABC.70–72
In 30% to 40% of cases, bony sclerosis is noted, with or without mottled destruction. Even though this radiographic appearance has occasionally been encountered in the metatarsal and phalanges, it seems to be fairly typical when the tarsal bones are involved. In this instance, underlying lytic change may not be apparent. A mixed lytic–sclerotic appearance or complete reactive sclerosis can occur. In the latter instance, there have been a number of case reports where this appearance has simulated chronic infection (in digits) or avascular necrosis (in the talus).73–76 Although sclerosis can radiographically mimic the denser ossific matrix patterns encountered in osteosarcoma, in Ewing tumor this reflects reactive bone formation.19,77,78 Therefore, radiographic differentiation with osteosarcoma may not be possible, as well as with some instances of chronic osteomyelitis.
Periosteal reactions are present in up to 50% of patients, especially in tubular bones. Classically, they appear as multilamellar “onion skin,” “lace-like,” or spiculated “hair-on-end” types. Nevertheless, it is important to note that, as with osteomyelitis, periosteal reactions are generally blunted when tarsal (cancellous) bones are involved.
Soft tissue mass is present in up to 90% of cases. Pathologic fracture may occur and be a presenting complaint.
Basic Differential Diagnoses
The main differential consideration for Ewing tumor is osteogenic sarcoma. Others include fibrosarcoma, reticulum cell sarcoma (non-Hodgkin lymphoma to bone), and osteomyelitis.
BASIC BENIGN BONE TUMORS AND TUMORLIKE CONDITIONS
Nonossifying Fibroma (Nonosteogenic Fibroma, Fibroxanthoma) and Fibrous Cortical Defect
These two benign fibrous lesions of bone most likely represent developmental defect rather than true osseous neoplasm; the literature considers them to be related to or in a “continuum” with one another. Both lesions typically are solitary and found within the metaphysis of growing tubular bones of children and adolescents. There is a decided predilection for long bones, especially the femur, tibia, and fibula. Although the majority of FCDs regress spontaneously, some evolve and enlarge into the medullary canal as the NOF. NOFs also spontaneously regress, usually by age 20. Both the FCD and NOF are histologically identical; they are also identical in that the pathologic stroma does not produce bone (unlike ossifying fibroma and fibrous dysplasia). By their very nature, however, they differ in their intraosseous location, clinical and epidemiologic data, and radiographic features.
Clinical Features
FCD is generally asymptomatic and discovered as an incidental finding. NOF is also generally asymptomatic, but may become painful with pathologic fracture or during spontaneous regression.
Epidemiology
FCD and NOF are very common. FCD generally comprises 5% of benign bone tumors, but this is clearly understated as most lesions are asymptomatic and therefore go unnoticed. The true frequency is more likely to be around 30% or greater of the normal population. NOF appears to be somewhat less commonly encountered; both lesions have been reported with a slight male preponderance. Up to 8% of people with NOF have more than one lesion, but the incidence may also be higher than reported. However, it is uncommon to have more than three tumors, which is usually indicative of an underlying syndrome or generalized disorder (e.g., Jaffe–Campanacci syndrome, neurofibromatosis). Sontag and Pyle79 identified this lesion in 54% of boys and 22% of girls in a series of 200 healthy children. These lesions occur in multiple sites in approximately 50% of patients.
Age Incidence
NOF is generally found during the first two decades, with a 13-year-old average age (it ranges between 4 and 40 years of age, albeit rare in the third decade). FCD is generally discovered between ages 4 and 8; it is rare under the age of 2 years and after age 14.
Location
The lower extremities are more common than the upper extremities. NOF and FCD are most often appreciated as incidental findings with ankle images. Despite the predilection for the lower extremity, with an incidence ranging from about 0.5% to 3.0% of primary pedal bone tumors, both lesions are extremely rare in the foot, although there have been reports of lesions in the midfoot and tarsal bones.19,78
Ritschl et al.80 radiographically classified these lesions into four stages:
Stage A: Oval lesion with smooth outlines
Stage B: Polycyclic borders
Stage C: Partially calcified/ossified lesion (progressive sclerosis)
Stage D: Completely sclerotic lesion
Radiographic Features of Fibrous Cortical Defect (Figure 20-13)
FCD is a round to oval, radiolucent intracortical/juxtacortical lesion that typically erodes the outer cortical surface. It is eccentrically situated, geographic, and its margin is sharp and sclerotic (type IA). The size is generally small, less than 3 cm (1–2 cm is typical). The lesion’s long axis is parallel to the diaphysis. FCD is found in the metaphysis of long bones; the distal tibia and fibula are quite common locations. The inner surface of the lesion’s outer “rind” may cause convexity of the internal cortex and impingement on the medullary canal. In the foot, a posterosuperior calcaneal tuberosity lesion has been noted growing away from the apophyseal region by the authors. FCD will spontaneously regress. Healed and regressed lesions may present as nontender, nonprogressive areas of focal cortical thickening.
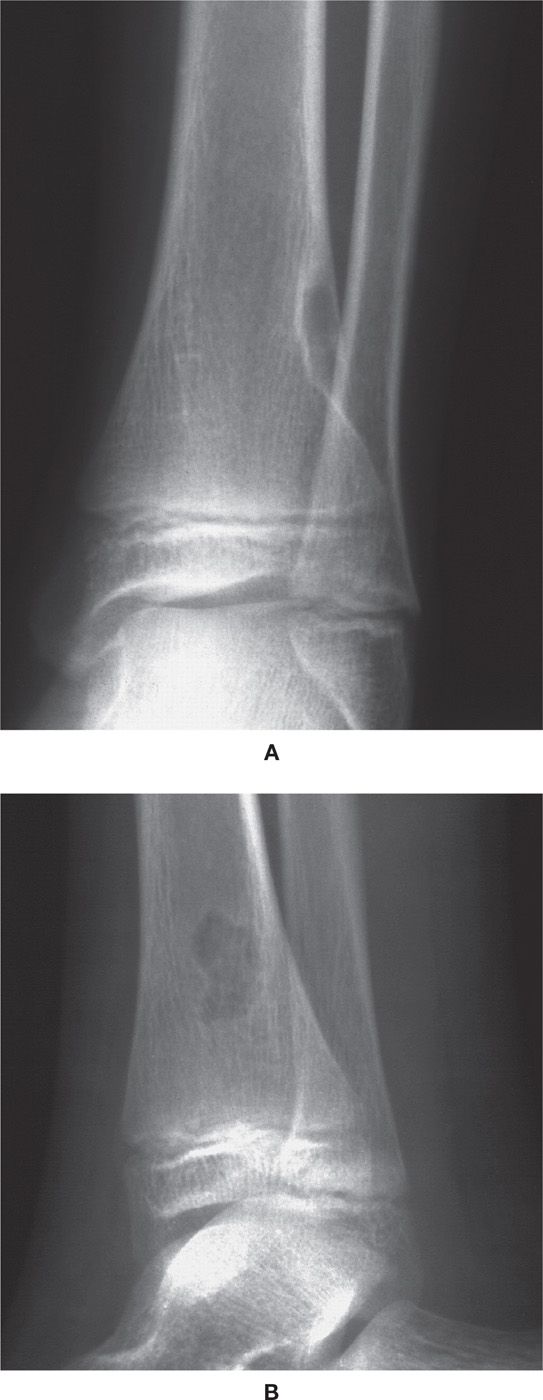
FIGURE 20-13. FCD in a 9-year-old male. A: Anteroposterior study of ankle reveals eccentric, oval metaphyseal lesion eroding the lateral cortical surface of the distal tibia. A well-delineated sclerotic reactive margin is obvious. B: Lateral ankle study of same lesion reveals polylobular outline.
Radiographic Features of Nonossifying Fibroma (Figures 20-10 and 20-14)
NOF is also a solitary, round to oval, geographic destructive lesion with a sharply defined sclerotic margin (type IA). Its position is metaphyseal to diametaphyseal and eccentric, often abutting the cortex on at least one radiographic view. The medullary margin of the lesion is sclerotic, with sharp outer definition. Just like the unicameral bone cyst, the lesion may grow longitudinally with the host bone into the diaphysis. The epiphysis is usually not involved. A multiloculated, “bubbly” (the so-called “bundle of grapes”) appearance is classic.
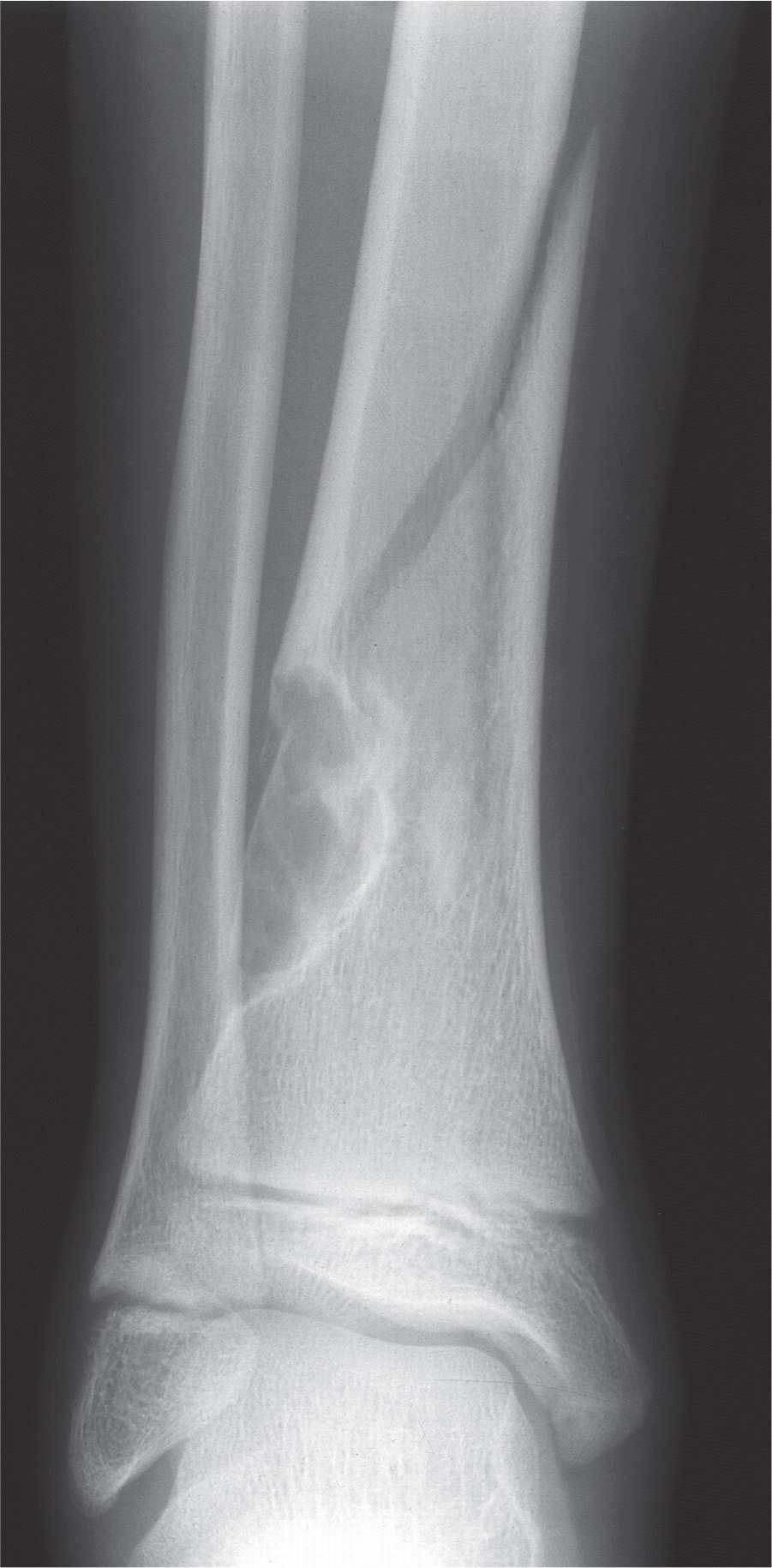
FIGURE 20-14. Evolution of FCD to an eccentric NOF has resulted in pathologic fracture. A spiral oblique fracture of the tibial midshaft can be seen emanating from the superior aspect of the lesion. The risks of pathologic fracture are increased with lesions that approximate 50% of the bony diameter.
In general, NOF lesions are greater than 3 cm and range from 4 to 7 cm. Distal tibial lesions can attain large size, which increases the risk of pathologic fracture. Lesions of the fibula commonly occupy the entire width of the medullary canal by the time of discovery, thereby appearing central. Mild eccentric remodeling and thinning is generally detected by a change in the external metaphyseal surface contour from concave to convex. Periosteal new bone formation is generally lacking, except in cases of pathologic fracture.
Pathologic fracture can occur when the lesion occupies 50% or more of the bone diameter.81 Symptomatic lesions suggest impending pathologic fracture, and in these instances, intervention may be considered.
Concave “scalloping” of the inner cortex favors diagnosis of NOF. Convex remodeling of the inner cortex (cortical “bubbling”) favors diagnosis of FCD.
As lesions resolve, lytic zones display progressive medullary sclerosis. Completely resolved and residual lesions assume the appearance of a focal, geographic osteosclerotic lesion of the metadiaphysis, often with lobulated outline.
Basic Differential Diagnoses
Although the radiographic findings are quite distinctive, the differential diagnosis includes CMF, solitary ossifying fibroma (osteofibrous dysplasia), and fibrous dysplasia. With regard to the latter two lesions, although rare, a number of cases of osteofibrous dysplasia have been reported affecting the mid-to-proximal diaphysis of the tibia (about 90% of lesions), with occasional ipsilateral fibular involvement. Lesions are typically eccentric, with a tendency to involve the anterior tibial cortex. The lesion of fibrous dysplasia is central and diaphyseal in location, unilateral, and classically demonstrates a “smudgy,” ground-glass appearance.
Solitary Osteochondroma (Osteocartilaginous Exostosis)
Solitary osteochondroma is a benign, slow-growing cartilaginous neoplasm that arises in close approximation to the growth plate of a long bone or in virtually any bone preformed in cartilage. Osteochondroma is considered to be the most common benign bone tumor. As its name implies, osteochondroma is an osseous outcropping from the surface of bone with a cap of hyaline cartilage, which generally varies in thickness with age. Osteochondroma is thought to arise via a herniation of physeal cartilage through the outer periosteal cuff (known as the encoche of Ranvier).82–84 Endochondral ossification takes place at the junction of cartilage and bone.85 The result is an exostotic lesion whose growth continues with that of the underlying bone, and whose growth ceases with closure of the physis. Rapid growth or continued growth after skeletal maturity should be considered signs suspicious of malignant degeneration. Although not common, sarcomatous degeneration of the cartilaginous cap has been reported (primarily to chondrosarcoma, occasionally osteosarcoma) occurring in less than 1% of the cases of solitary osteochondroma.85–87
There is a skeletal dysplasia that presents as a multiple form of osteochondroma known as hereditary multiple exostoses, multiple osteochondromatosis, or diaphyseal aclasis. Radiographically, the most striking feature of hereditary multiple exostoses is the presence of many exostoses along with growth disturbances that often result in shortened and flared tubular bones. In this disorder, involvement of the epiphyses (rather than the epiphyseal plates) occurs.88,89 From a pedal perspective, these lesions tend to involve the metatarsals, proximal phalanges, and distal tibia and fibula.
Unless calcified, the cartilaginous cap of an osteochondroma is not radiographically discernable. However, both CT and MRI are able to accurately assess the thickness of the cartilaginous cap. Cartilaginous caps are typically thin or absent in adults, especially in nonprogressive lesions. As a rule, cap thickness greater than or 1.0 to 1.5 cm should be carefully evaluated to rule out secondary chondrosarcoma. At 2 cm or greater, malignancy is suspect.90
Two basic morphologic types of osteochondroma are encountered: pedunculated and sessile. The pedunculated osteochondroma is mushroom-shaped, with a slender osseous stalk that connects the cap to underlying bone. The majority of osteochondromas are of the pedunculated variety. The sessile osteochondroma appears flared or has a broad-based attachment that appears rounded, lobulated, or “plateau-like” in shape. Sessile osteochondromas tend to be more deforming and have a higher incidence of malignant degeneration.
Location
Osteochondromas typically arise from the metaphysis and then continue to grow until bony maturity. In this regard, they can follow longitudinal bone growth into the diametaphysis. Epiphyseal locations are exceedingly uncommon and suggest the diagnosis of dysplasia epiphysealis hemimelica or Trevor disease.
Bodywide, there is a predilection for the lower extremity, especially distal femur. The tibia is involved in about 20% of cases. Although they may be found in any bone preformed in cartilage, true osteochondroma is not commonly encountered in the distal extremities. They have been described emanating from the digits, metatarsals, calcaneus, dorsal talus, as well as the distal tibia and occasionally the fibula. The most common site is generally given to be the metaphysis of the phalanx (approximately 75%), with the metatarsal shaft also considered to be a relatively common site. However, the majority of digital lesions emanating primarily from the dorsomedial aspect of the hallucal distal phalanx are typically the subungual variant and not a true osteochondroma.
In addition, with respect to foot and ankle involvement, osteochondroma has been reported in the cuneiforms, distal tibia and fibula, and rarely in the calcaneus and talus.19,78,91,92 Both talar neck and body lesions have been reported. Although calcaneal lesions are rare, it is interesting to note that there have been several case reports involving the peroneal and plantar tubercles.93–98
Clinical Features
Osteochondroma is generally asymptomatic but presents as a slow-growing “bump” or “growth.” Signs and symptoms are often related to mechanical pressure, cosmetic deformity, overlying bursa formation, neurovascular impingement, fracture, or malignant transformation. Subungual variants may be discovered in the workup relating to chronic paronychia or in suspected nerve compression. A bursa occasionally develops over the cap portion of the lesion. Rarely, first metatarsal osteochondroma may present as an enlarging hallux valgus deformity. Although this seems paradoxical inasmuch as there is no growth plate in the area, one must remember that double metatarsal epiphyses are a relatively common finding in the pediatric population.
Epidemiology
Osteochondroma is the most common benign bone tumor (approximately 35%, ranging from 20% to 50%)99–102 and comprise about 10% all bone tumors. The male-to-female ratio is 1.5 to 2:1. Nearly 75% of patients present in the first two decades of life, and most lesions are diagnosed by the age of 30. Despite these impressive numbers, the frequency of solitary osteochondroma in the foot is not high, with numbers ranging from 0.5% to 1% of all osteochondromas (approximately 5% of all primary tumors of the foot).46,89,102–104
Radiographic Features (Figures 20-15 and 20-16)
The protruding exostosis comprises of both cortical and medullary bone. It has either a constricted pedicle (pedunculated) or broad (sessile) base emanating from the underlying cortex, which is typically is flared. Its position is predominantly metaphyseal, although the diaphyseal cortex occasionally flares into the osteochondroma. Epiphyseal osteochondroma is rare and suggests the diagnosis of Trevor disease. Multiple views are necessary to optimally demonstrate medullary confluence with the host bone. When equivocal, CT is used to definitively establish blending of the cortex and medullary canal with the underlying bone. There is a tendency for the stalk to be directed away or grow away from the adjacent physis in skeletally immature patients, or from the nearest joint toward the midshaft in the adult. Growth ceases with closure of the physis. Resorptive or erosive change, rapid enlargement, or cap calcification may reflect malignant degeneration to chondrosarcoma.
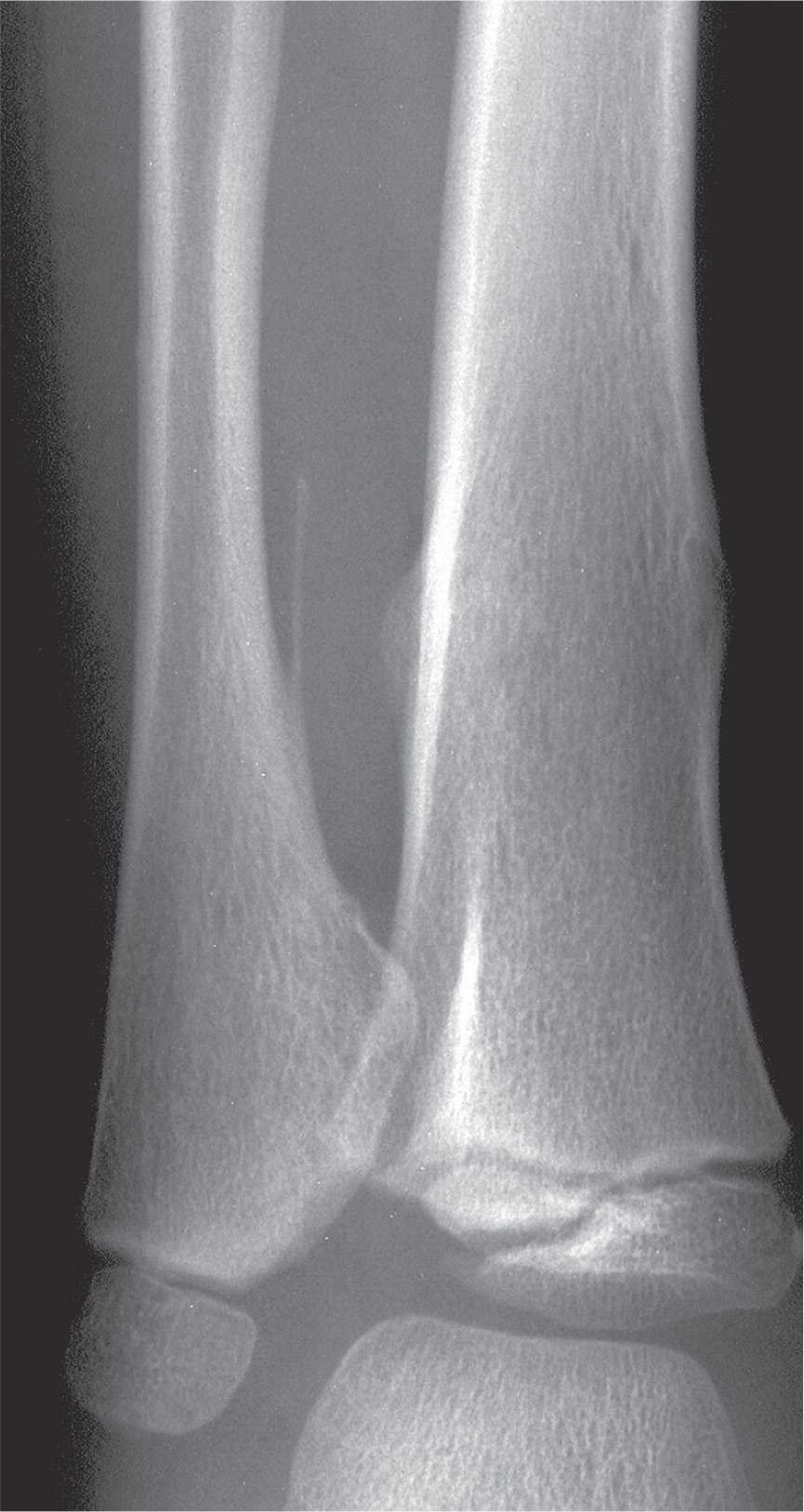
FIGURE 20-15. Multiple hereditary exostoses, tibia and fibula. A pedunculated osteochondroma arising from the medial apect of the distal fibula diaphysis demonstrates a thin, long stalk and projects superiorly. A sessile osteochondroma is seen along the lateral aspect of the distal tibia diaphysis at the same level as well as along the medial aspect of the distal fibula diametaphysis.
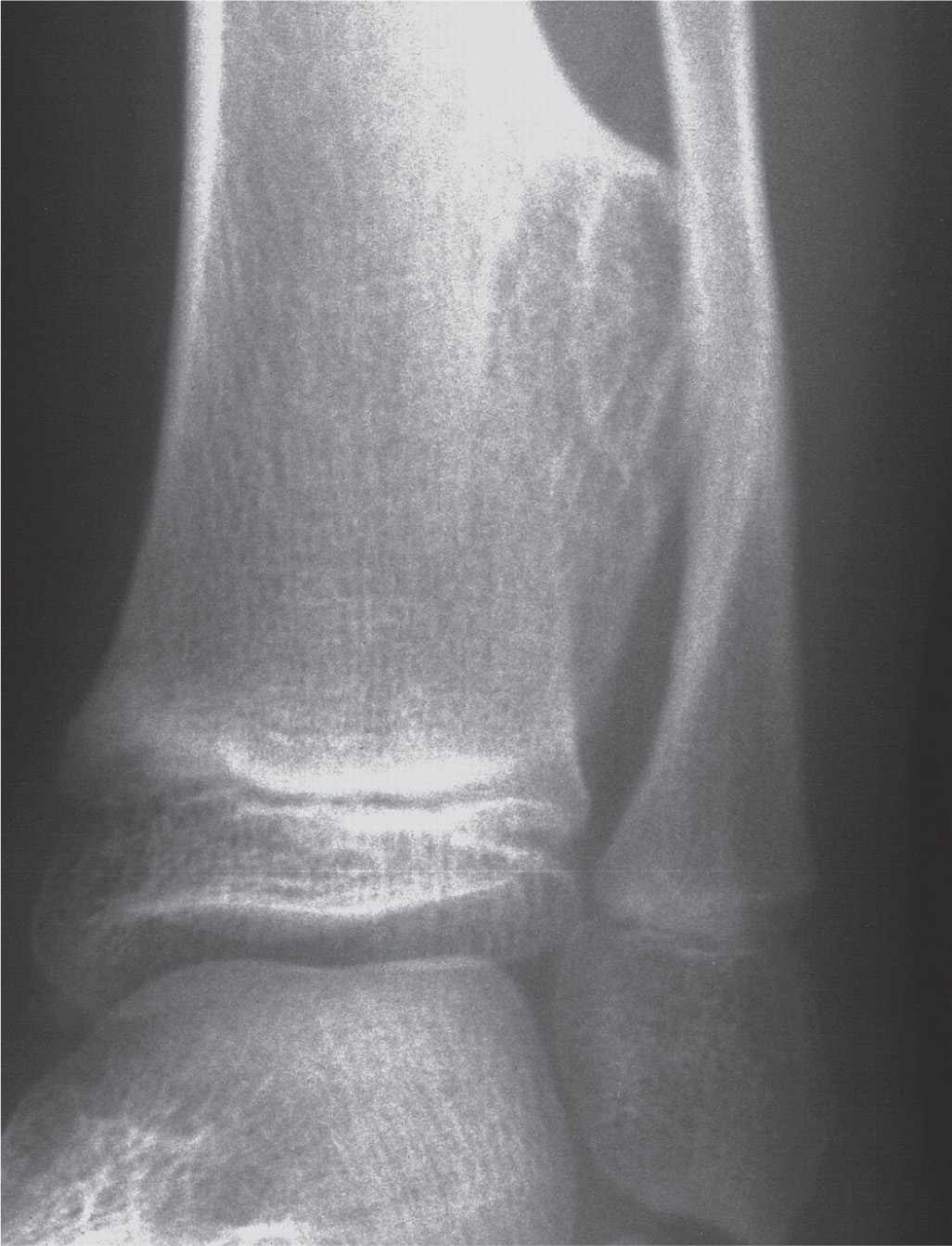
FIGURE 20-16. Sessile osteochondroma, tibia. Broad-based, plateau-shaped lesion involving the distal lateral tibial metaphysis–metadiaphysis of a 7-year-old female. The cortex is flared, and medullary confluence is obvious. A mild angular deformity of the distal tibia is noticeable. Consistent pressure of this slow-growing lesion has resulted in “saucerized” remodeling of the adjacent cortex of the distal–medial fibula along with lateral bowing of the fibular diaphysis. Note: The edges of a broad-based exostotic lesion viewed in line with its axis may impart the erroneous appearance of a geographic type IA margin to a pseudolytic lesion. Multiple views should always be obtained.
Lesions are commonly larger than they appear radiographically secondary to the noncalcified cartilaginous cap.105,106 Occasionally, the cartilaginous cap may demonstrate punctate calcifications or irregular ossifications.67,100 Growth of the lesion ceases with that of the underlying bone. Continued growth and a hyaline cartilage cap greater than 1.5 to 2.0 cm in thickness after skeletal maturity suggests malignant transformation (most frequently to chondrosarcoma).67,83,90
Distal tibial and fibular lesions approximating the location of the syndesmosis are commonly quite deforming by the time the patient presents with ankle symptoms. The bony segment contiguous to the osteochondroma cap is typically under pressure, resulting in resorption and remodeling.
Subungual Exostosis
Originally described in 1847 by Dupuytren,107 subungual exostosis is considered a variant of osteochondroma. As the name indicates, the bony projection arises from the terminal phalanx. It mimics osteochondroma, but differs in several key epidemiologic, radiographic, and pathologic aspects. Specifically, and as noted before, subungual exostosis is far more commonly encountered in the foot.
Radiographically, subungual exostosis may appear pedunculated, but, unlike true osteochondroma, it lacks corticomedullary continuity (Figure 20-17). In this regard, it is radiographically similar to bizarre parosteal osteochondromatous proliferation (BPOP) and other posttraumatic reactive periosteal lesions. It should be differentiated from the subungual spur that is associated with hallux limitus, presumably the result of chronic soft tissue compression (with or without shoe-box trauma). This lesion emanates from the most distal–dorsal aspect of the terminal tuft and is almost never constricted.
Trauma has been implicated in the formation of subungual exostosis, although it is unclear what role hematoma plays. It is clear that local proliferation of fibroblasts occurs early in the process. These cells then undergo cartilaginous metaplasia, which, in turn, leads to progressive ossification. Histologically, subungual exostosis possesses a fibrocartilaginous cap in contrast to hyaline cartilage for true osteochondroma. Therefore, subungual exostosis represents a distinctly different pathologic entity. Moreover, malignant degeneration has not been reported.
Bizarre Parosteal Osteochondromatous Proliferation (Nora Lesion)
BPOP is an exostotic lesion generally derived from the parosteal tissues with a high frequency for the small bones of the hands and feet.108 The lesion consists of cartilage, bone and fibrous tissue, and often has a cartilaginous cap. This lesion probably represents one of the spectra of posttraumatic cortical lesions (florid reactive periostitis, turret exostosis, subungual exostosis) derived from subperiosteal hematoma and/or inflammatory changes in and/or around the periosteum. Pathologically, nuclear atypia is common in the cartilaginous components, thereby simulating malignancy.
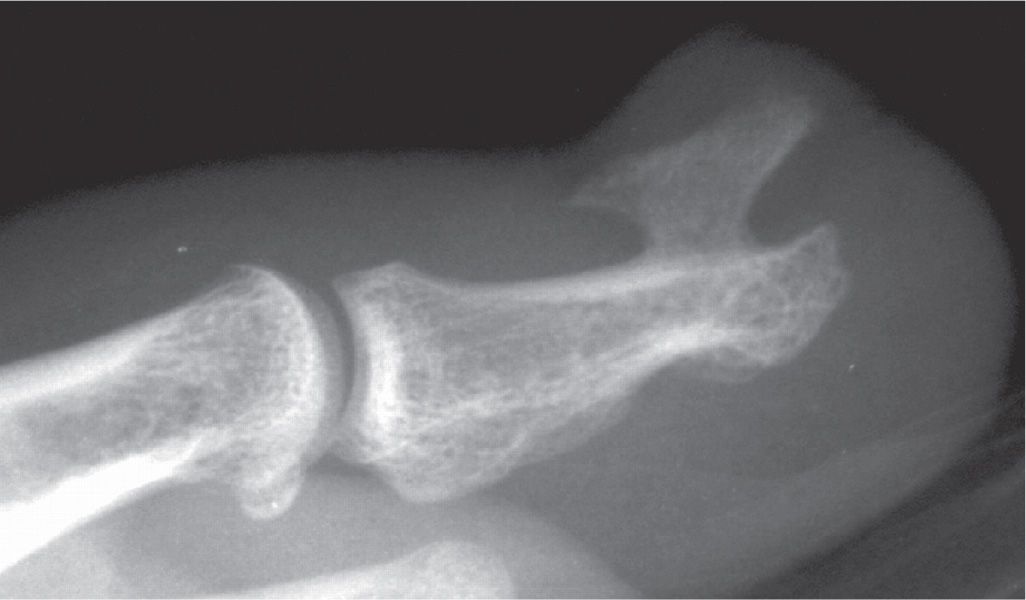
FIGURE 20-17. Subungual exostosis, hallux. Lateral study of hallux demonstrates a large, dorsal and subungual exostotic lesion without medullary confluence or any evidence of corticolesional flaring. The odds are against this being a true osteochondroma.
Clinical Features
The male-to-female incidence is 1:1; most cases are discovered in an age range from the mid–third decade to the fourth decade (slightly older than osteochondroma). Patients predictably complain of a painful dactylitis, generally without a history of trauma or underlying arthropathy. Virtually all cases involve the small bones of the hands and feet.
Radiographic Features
BPOP mimics osteochondroma, but with several significant differences: (1) it is a well-defined ossified mass arising from the cortical surface; (2) it may appear pedunculated, attached by a pedicle; (3) flaring of the underlying bony cortex is absent; (4) medullary confluence is absent (a critical point, which may require ancillary CT or MRI to positively establish; and (5) a periosteal reaction is typically present.
Simple Bone Cyst (aka Unicameral or Solitary Bone Cyst)
The simple bone cyst is a nonneoplastic, fluid-filled cavity lined by a thin fibrovascular connective tissue membrane and/or osteoid; their location is intramedullary. Cyst cavities may contain straw-colored serous, serosanguineous, or frank blood when pathologic fracture occurs.
Epidemiology
This lesion arises in growing long bones; it is relatively common in childhood and early adolescence, with a peak incidence during the first two decades. However, the occurrence of simple bone cyst in the foot is rare, and the midbody of the calcaneus is the most common site, approximately 4% of all cysts. The male-to-female ratio equals 2:1 to 3:1.
Clinical Features
Most lesions are asymptomatic discoveries, unless microfracture or pathologic fracture occurs. Contrary to cysts of long bones, pathologic fracture of calcaneal lesion is not common. Even though the calcaneus is a weight-bearing bone, the unique architecture of its neutral triangle results in low physiologic stresses.109 Limitation of joint motion has been reported, as well as pain and swelling in tubular bones, but these are less common presentations.
Location
Metaphyseal and, less commonly, diaphyseal regions of the proximal humerus and femur are frequently targeted locations. Bone cyst is less commonly encountered in the tibia and fibula. In long bones, the simple bone cyst typically abuts against the metaphyseal side of the physis and demonstrates “centripetal” longitudinal extension into the diaphysis. In general, growth of this lesion ceases with longitudinal growth. According to the well-known Neer classification,110,111 unicameral cysts in long bones that are located at a distance from the cartilaginous growth plate are considered inactive. Those in direct contact with the growth plate are considered active. Unfortunately, the applicability of the Neer classification to unicameral bone cyst of the calcaneus (and other bones preformed in cartilage) is unclear.
As previously noted, the neutral triangle of the os calcis is a primary pedal site. Occasionally, simple bone cyst is encountered in tarsal and short tubular bones, especially the metatarsals and, occasionally, phalanges.19,112,113 When a cyst-like lesion is encountered in the phalanx, the occurrence of simple bone cyst is sufficiently rare, meriting consideration of other conditions such as epidermoid inclusion cyst, intraosseous ganglionic cyst, enchondroma, and intraosseous glomus tumor. Penetration of the growth plate and epiphyseal extension is unusual, but has been described.114 Much like osteochondroma, the simple bone cyst tends not to progress after skeletal maturity, and spontaneous regression has been reported.
Radiographic Features (Figures 20-18 and 20-19)
The simple bone cyst is a solitary, centrally located geographic (oval-shaped) lesion of the long bone metaphysis, metadiaphysis, or diaphysis. It classically demonstrates a sharply delineated margin and a thin, well-delineated sclerotic halo. This is consistent with type IA growth rate assessment. As previously noted, epiphyseal involvement is uncommon. The simple bone cyst usually appears unilocular, occasionally multilocular, and without endosteal scalloping. It may be moderately expansile in tubular bones, but never penetrates the cortex; therefore, there is no soft tissue extension. Simple bone cyst of the calcaneal neutral triangle is a central type IA to IB marginated lesion with relatively straight anterior and rounded posterior edges.
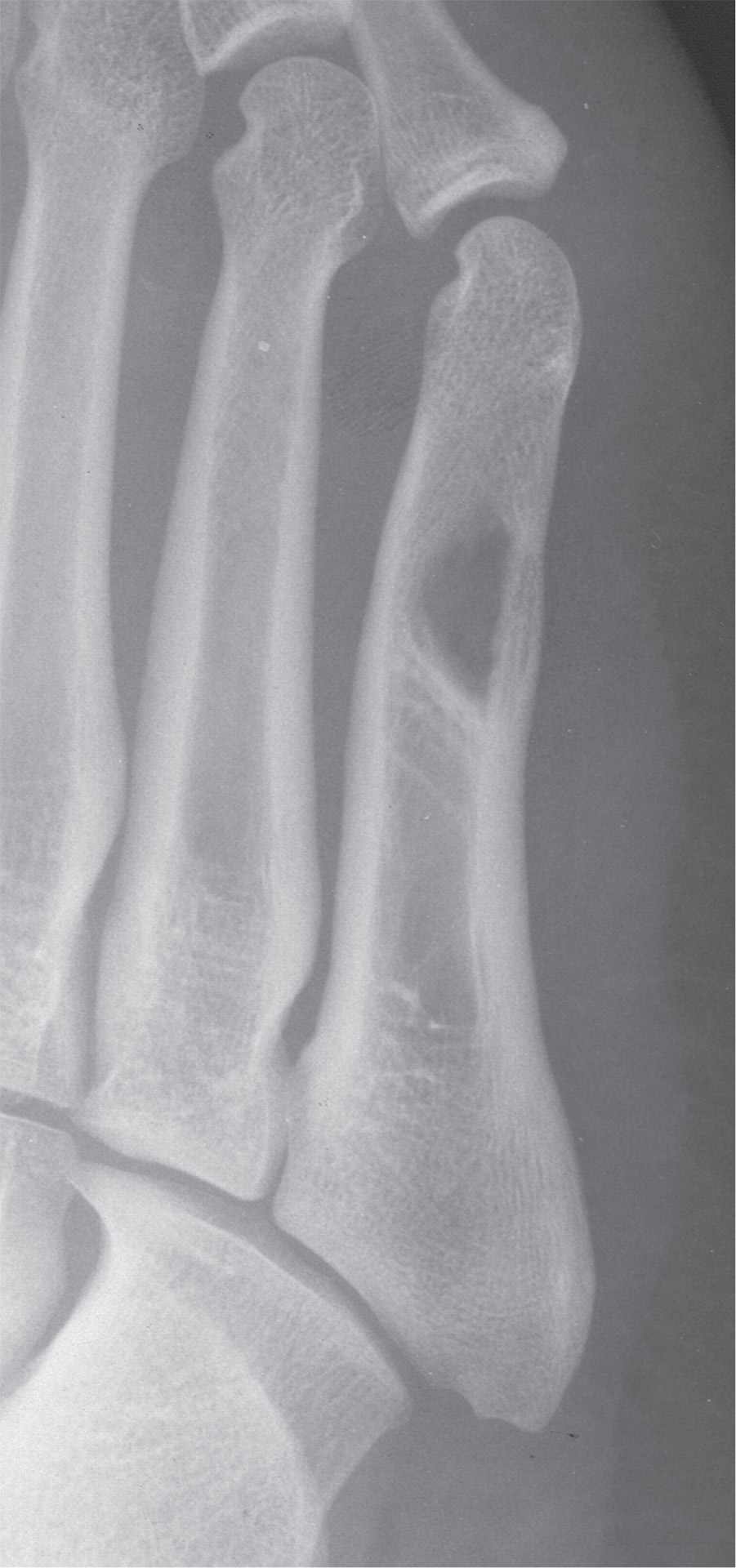
FIGURE 20-18. Simple bone cyst, fifth metatarsal. A geographic lytic lesion with no sclerotic margin (type IB) is seen in the fifth metatarsal diaphysis. The paient had pain in the area; histology confirmed the diagnosis. (Courtesy of D. Scot Malay, DPM, Philadelphia, PA.)
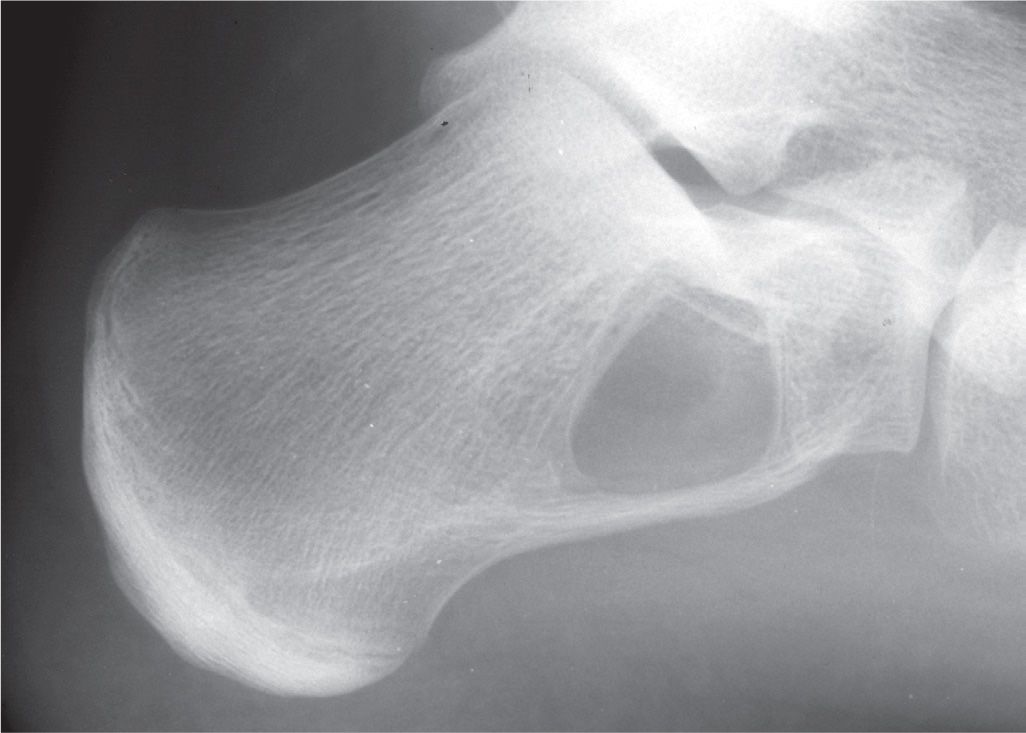
FIGURE 20-19. Simple bone cyst, calcaneus. This 16-year-old demonstrates a geographic lesion with sclerotic margin (type IA) in the calcaneal neutral triangle.
Periosteal new bone formation is unusual and generally indicates pathologic fracture; this typically occurs with large lesions. Ossific fragments are known to gravitate to the bottom of the cyst in long bones (“fallen fragment” sign), which is considered pathognomonic.115
Pathologic fracture is unusual in calcaneal lesions, but it can occur and has been reported with very large lesions.116–118 In a study of 50 simple bone cysts in 47 patients, Pogoda et al.118 determined a critical cyst size to be when it occupies 100% of the coronal plane width (best determined with CT scanning) and at least 30% in the sagittal plane.
Differential Diagnosis
In the more common calcaneal location, pseudocystic neutral triangle and ABC should be considered. In older patients, other differentials include intraosseous lipoma and rare, aggressive vascular lesions such as hemangioendothelioma and hemangiopericytoma.119
Aneurysmal Bone Cyst
Not a true neoplasm but probably a reactive process (to trauma or another underlying bone lesion), ABC is a rapidly expansile bone lesion composed of multiple blood-filled cystic cavities; their walls are composed of fibrous tissue and various amounts of osteoid, chondroid, and multinucleated giant cells. The lesions are benign, but often locally aggressive. ABC can be primary, but are most often seen in association with GCT (19%–39%); other common associated lesions include angioma, osteoblastoma, and chondroblastoma. Less common precursor lesions include CMF, NOF, solitary bone cyst, and eosinophilic granuloma.120 Association with osteosarcoma has also been described.
Epidemiology
As with most bone tumors, occurrence of ABC in the foot is rare. Approximately 5% to 9% of all ABCs arise within the foot, and comprise about 6% to 8% of all foot tumors.78,121,122 In a fairly recent large series by Chowdhry et al.123 that was performed over a 25-year period, 155 cases out of 273 involved the lower extremity. Of these, 18 cases (6.6% of all ABCs) involved the foot. In a retrospective study of 150 ABC patients over a 20-year period, Mankin et al.124 noted 7 cases in the distal tibia (4.6%), 7 cases in the distal fibula (4.6%), and 8 total cases in the foot (5.3%), or about 10% involving the foot and ankle. With respect to age, about 80% occur within the age ranges of 5 to 20 years, and up to 90% of lesions are encountered in patients less than 30 years old.48,61,119,125–127 Lesions are rarely encountered in infancy, in children less than 5 years old, or after the fourth decade. Females are slightly more frequently affected than males.
Clinical Features
There is rapid onset of pain and swelling. Limitation of joint motion is not uncommon, and dactylitis is typical. Pathologic fracture is possible.
Location
Primary lesions can involve almost any solitary site; the most frequently involved areas are the vertebrae and the metaphyses of long bones, especially around the knee. There may also be an increased tendency to involve the distal fibula, and up to 15% may involve the small tubular bones of the hands and feet.128 In the foot, most cases of ABC involve the small tubular bones, especially the metatarsals. However, there are numerous reports of ABC involving the calcaneus and talus. In one of the larger series based on a 44-year retrospective review of ABC and GCT of the foot from the Rizzoli Institute, of 24 total cases of ABC, Casadei et al.126 noted 8 in the metatarsals and phalanges as opposed to 10 in the os calcis and 5 in the talus. When they added their data to that of the available literature at that time, of 68 pedal cases of ABC, 39 involved the talus and calcaneus as opposed to 25 for the metatarsals and phalanges. Although rare, the lesion has been reported in the navicular and the cuboid, though a number of these are secondary lesions.17,20,61,78,119,121,125–145
Radiographic Features (Figures 20-20 and 20-21)
The classic radiographic description of an ABC cyst is that of an eccentric, geographic, and metaphyseal lytic lesion that may either be purely lucent or display trabeculation(s) in a skeletally immature patient. Though the tumor is typically found eccentrically, it may be centrally located in the fibula or small tubular bones of the foot. In the calcaneus, ABC tends to arise posteriorly and/or inferiorly.78
When coarsely trabeculated, the appearance has been likened to that of a “soap bubble” (the stable phase). When expansile, the original periosteum is often lifted from the cortex; this then forms a cuff or buttress of bone at the angle between the original cortex and the expansile portion. Although the term buttress is applied, this should not be confused with the interrupted solid periosteal reaction that is also referred to as a buttress periosteal reaction.
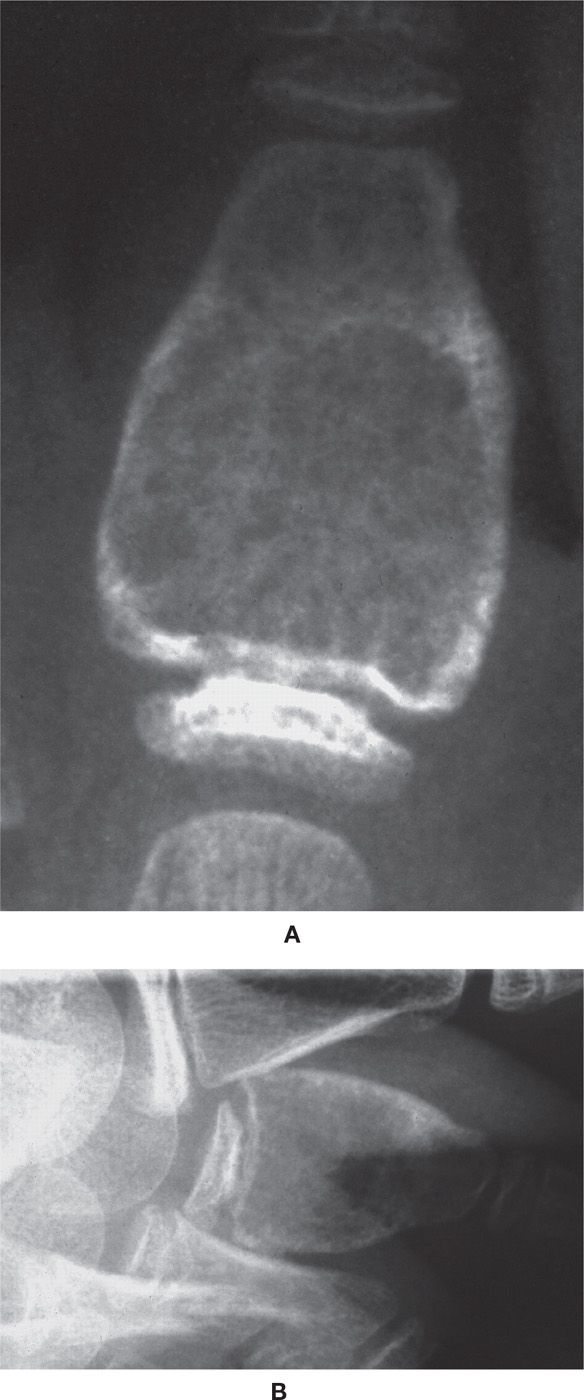
FIGURE 20-20. Aneurysmal bone cyst, phalanx. A: Anteroposterior view. B: Lateral view. A nonmatrix-forming, expansile geographic lesion involving the proximal metaphysis and the entire diaphysis of a second proximal phalanx in an 8-year-old female patient. Expansion (cortical replacement) is concentric and has produced the appearance of a finely pseudoseptate “shell.” In the absence of adequate imaging, the septa can appear smudgy or resemble ground glass. The appearance of an open epiphysis and a nonepiphyseal location argue against GCT and in favor of an ABC.
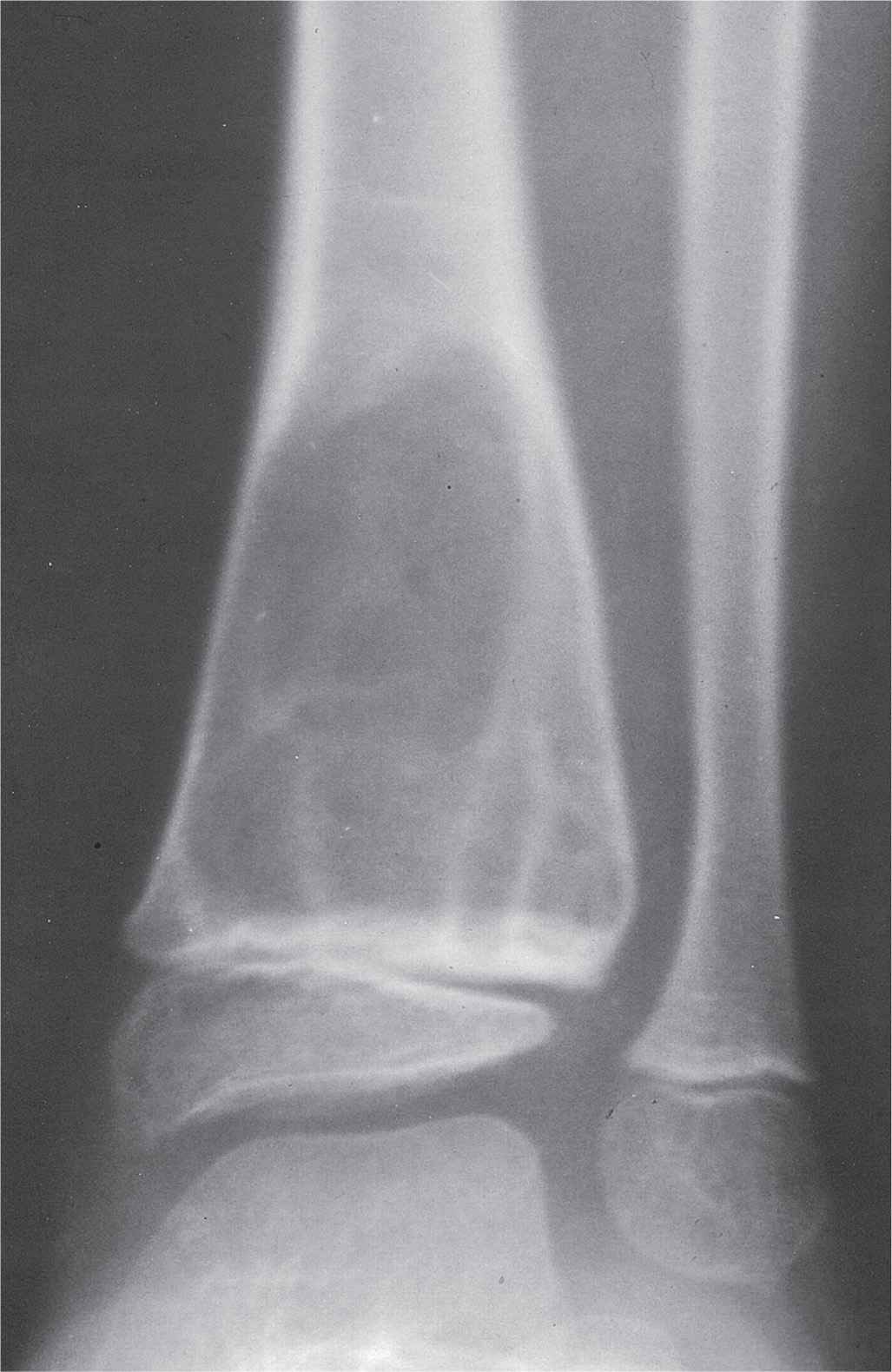
FIGURE 20-21. Aneurysmal bone cyst, distal tibia. The geographic type IB lesion is centered in the metaphysis and stops at the physis of this child. Trabeculations are present throughout this histologically confirmed lesion. (Courtesy of John Walter, DPM, Philadelphia, PA.)
The radiographic appearance generally corresponds to the aggressiveness of the lesion. Wilner127 and Fechner and Mills146 have recognized radiographic stages of ABC (Table 20-5). Margination generally correlates with the stage of the lesion. The growth rate of most ABCs is type IA (approximately 30%) or IB (approximately 60%). However, a type IC destructive pattern may also be encountered in 10% to 15% of those rapidly expanding lesions.78,120
ABC appears to be statistically less likely to present with asymptomatic pathologic fracture as opposed to unicameral bone cyst. This is not secondary to any particular mechanical property of the ABC, but rather the early development of pain with most lesions.137
Consistent with the blood-filled cavities that characterize ABC, intralesional fluid–fluid levels are frequently encountered in CT and MRI images. This is presumably the end result of red blood cell layering secondary to gravitation.
Radiographic Nuances
ABC does not produce an internal matrix; therefore, no internal ossification or calcification will be seen. The “finger-in-balloon” sign represents the original cortex protruding into the markedly expanded lytic zone; this finding is usually not encountered with GCT or simple bone cyst. Codman angle may be seen during rapid growth phases; however, an underlying aggressive periosteal reaction is generally not identified. In long bones, septation is often not uniformly packed and somewhat coarser than GCT.
There is no propensity for ABC to spread to the subarticular cortex or cross the physis; however, cortical breakthrough with soft tissue invasion is possible. ABC may extend into the epiphysis after physeal closure, but this is not a direction of preferential growth; epiphyseal extension has rarely been reported with an open physis.17,121,147,148
Secondary ABC typically resembles the precursor lesion. Although secondary ABC formation is most commonly noted with benign lesions such as GCT and CMF, it is worth noting that secondary ABC is occasionally associated with various malignant lesions such as osteosarcoma, chondrosarcoma, fibrosarcoma, and angiosarcoma.149–152 Several cases of calcaneal osteosarcoma have been reported to radiographically mimic ABC.153
Differential Diagnosis
Albeit rare in the foot, simple bone cyst, hemangioma, desmoplastic fibroma, and telangiectatic osteosarcoma can radiographically simulate ABC. However, the four major differential considerations for an ABC in pedal imaging are GCT, CMF, enchondroma, and solid variant (giant-cell reparative granuloma). As enchondroma develops, it can generally be differentiated based on endosteal scalloping, eccentric lobulation, cartilaginous matrix, older age, and so on.
Giant-Cell Tumor of Bone (Osteoclastoma)
An uncommon tumor of marrow connective tissue origin, it appears to be more common in the foot than once thought. Histopathologically, multinucleated giant cells (hence the alternate name osteoclastoma) against a background of mononuclear spindle-shaped stromal cells characterize GCT.154 The ratio of stromal to giant cells is related to the lesion’s aggressiveness.
| Radiographic Staging of Aneurysmal Bone Cyst |
Wilner127 | Fechner and Mills146 | Description |
Stage I | Stage I: Incipient | Small, eccentric, geographic (type IA or IB margins) lesion with no expansion. Periosteal elevation typically present. Patients are often asymptomatic |
| Stage II: Growth | Rapid destruction with cortical lysis, no perceivable cortex; type IC margination common. Classic “blow-out” appearance |
Stage II | Stage III: Stablization | Classic picture: maturation of shell and progressive thickening of trabeculation leads to “soap-bubble” appearance. Thin surrounding cortical shell overlaps normal diaphyseal cortex, so-called “finger-in-balloon” sign |
Stage III | Stage IV: Healing | Progressive ossification of lesion with transformation into an irregular, dense, coarsely trabeculated mass |
Multicentric GCT has been reported to occur either idiopathically or arising in Pagetic bone. When idiopathic, onset may be synchronous or metachronous. Additionally, there appears to be an increased incidence of small bone involvement in the hands and feet (especially the hands).155,156
Epidemiology
GCT is benign, but often locally aggressive. With a prevalence of less than 2%, GCT is considered rare in the hand and foot bones.155,156 Unni157 reports an incidence of 1.7% in the hand and 1.2% in the foot. Huvos17 reports a higher incidence in the hand (3.75%) and only 1.8% in the foot. Likewise, Mirra et al.158 report an incidence of fewer than 4% in the hand and less than 2% involving foot bones. Conversely, in a 50-year review of almost 900 cases of GCT at the Rizzoli Institute, Biscaglia et al.159 reported more foot than hand cases. Specifically, they found 21 cases in the foot bones (approximately 2.3%) and 8 in those of the hand (less than 1%).
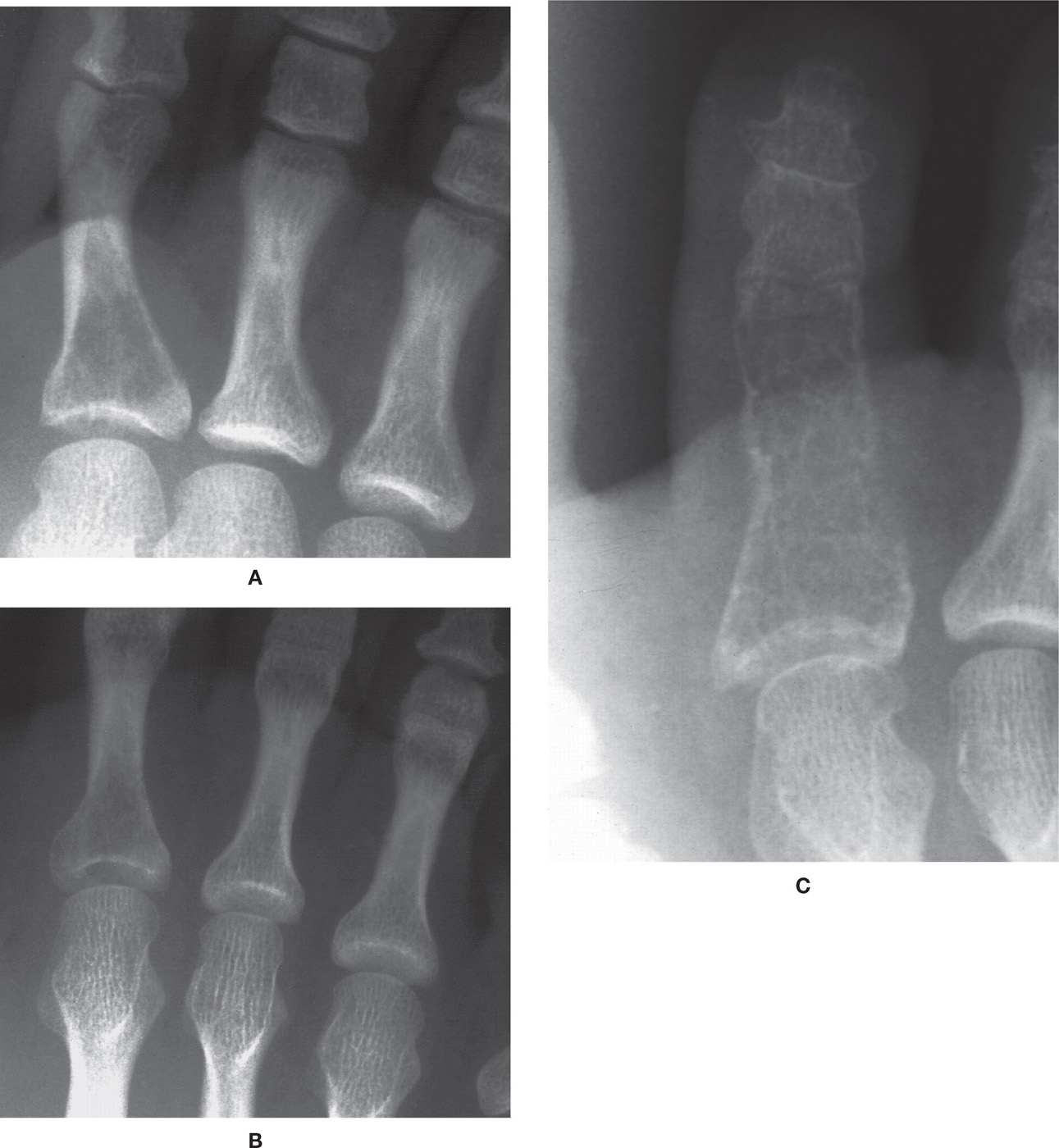
FIGURE 20-22. Giant-cell tumor, phalanx. A 31-year-old visits a local urgent care center after stubbing second toe. A: Medial oblique view demonstrates hyperlucency of second proximal phalangeal base, extending from the subarticular cortex. The lesion is poorly delineated, suggestive of geographic IC type margins, and virtually invisible on dorsoplantar (DP) study. B: Differential diagnoses include intraosseous hemorrhage and GCT. Nevertheless, these radiographs are read as normal. C: Same patient 9 months later. Virtually the entire cortex of the proximal phalanx has been replaced by a pseudoseptated periosteal shell. Trabeculation is uniform. No hint of lobulation or endosteal scalloping is apparent. Final pathologic diagnosis after amputation was aggressive GCT.
Seventy-five percent occur within the age range of 15 to 40 years (with a mean in the fourth decade); they almost always occur in a skeletally mature individual. Fewer than 3% of cases occur before 14 years of age; only 13% of cases occur in patients over the age of 50 years. In the Casadei et al.126 series, the average age of patients affected by ABC was 15 years as opposed to 27 years for GCT. Recent studies now indicate an increased prevalence among females, especially in the minority of GCTs that occur before skeletal maturity.160 GCT is rare in blacks and occurs at a higher rate in people of China and Southern India.161,162
Clinical Features
The chief presenting symptom is a dull ache, possibly associated with a palpable, tender mass. In the toes, dactylitis is typical. Adjacent joints are commonly symptomatic. Pathologic fracture is probably not as frequent as reported in some studies (inasmuch as the lesion typically breaks through the cortex), and varies from 10% to 35%.
Location
The knee is involved in up to 50% of patients, followed by the distal. Of those that are found in the foot and ankle, the distal tibia represents at least 80% of reported cases. In the foot, the phalanges (primarily the proximal phalanx), metatarsal bones (especially the first metatarsal base and lesser metatarsal heads), talus, and posterior calcaneus are the sites reported most. When the phalanx is involved, it is typically the proximal portion. However, some studies indicate that the talus and calcaneus are more frequent than the forefoot bones.126,159 In the composite study by Casadei et al.,126 ABC was much more common in the small tubular forefoot bones, whereas GCT was more common in the small midfoot tarsal bones. Frequencies in the talus and calcaneus were fairly close, with ABC slightly more common in the calcaneus (57% vs. 43%) and GCT more common in the talus (55% vs. 45%).126 A few cases of midfoot/lesser tarsal bones have been reported.163 Yanagisawa et al.164 found that when the small bones of the hands and feet were affected, the patients were younger. In tubular bones, GCT is characteristically an eccentric lesion of the metaphysis (epicenter) with subsequent centrifugal epiphyseal extension to the subarticular cortex.
Radiographic Features160,165,166 (Figures 20-7 and 20-22 to 20-25)
GCT typically presents as an eccentrically situated, geographic lytic lesion with a nonsclerotic margin (type IB). Lesions arise in the metaphysis, but with a decided propensity for centrifugal spread in long bones; as a result, the lesion can extend to subchondral bone and subarticular cortex.
In small tubular bones, GCT often presents as a rapidly expansile radiolucent lesion. Although classically eccentric, as a metaphyseal lesion it spreads to the subarticular cortex, quickly changing its appearance in the foot. (Though there is marked propensity for spread to the subarticular cortex, it rarely crosses the joint or the cartilaginous open physis.) Eccentricity of the lesion is encountered, but only early in its course. Relatively quickly, GCT tends to occupy much of the bone; therefore, central location is typical in the digits, metatarsals, and even talar body by the time of discovery.
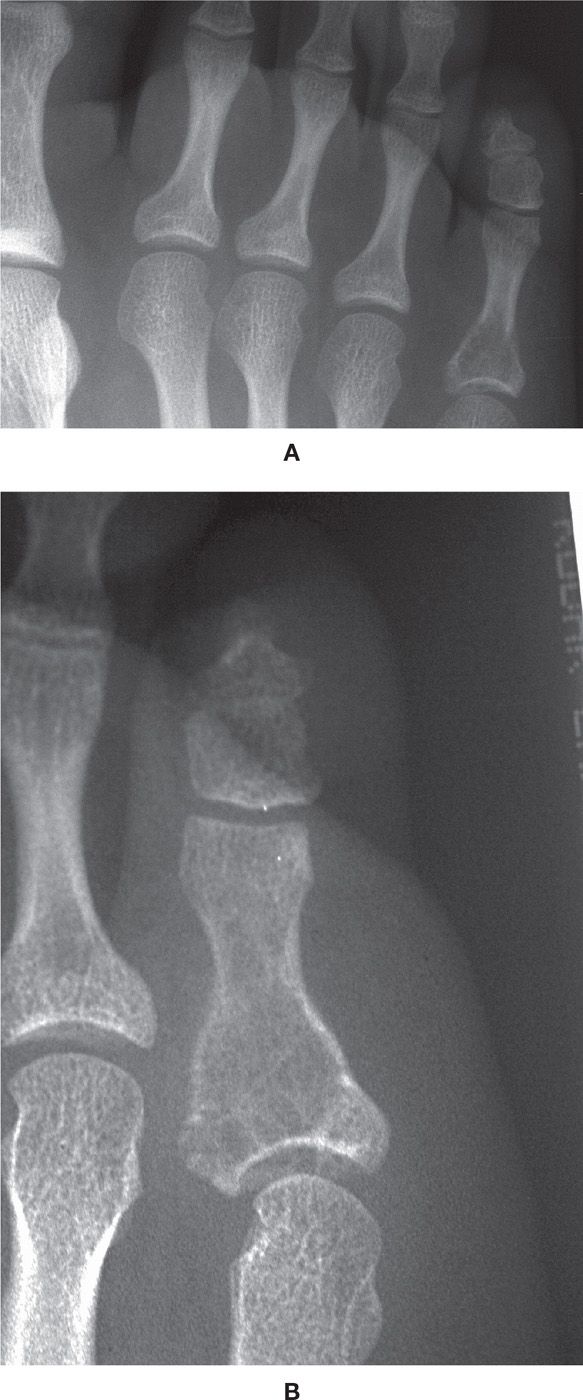
FIGURE 20-23. Giant-cell tumor, proximal phalanx. A: DP view. B: Medial oblique view. This 15-year-old female patient (with closed physes) presented with insidious onset of a painful fifth toe. Radiographs demonstrate a geographic lytic lesion with poorly defined, nonreactive margins (type IC) of the proximal phalanx; it is centered in the base (metaphysis). The lesion is clearly expansile (the cortex has been replaced) and has a finely trabeculated shell. There is extension such that the lesion occupies the entire subarticular cortex proximally; it also extends distally into the proximal diaphysis. This case reinforces the principle that it is the presence of an open physis that argues strongly against the diagnosis of GCT.
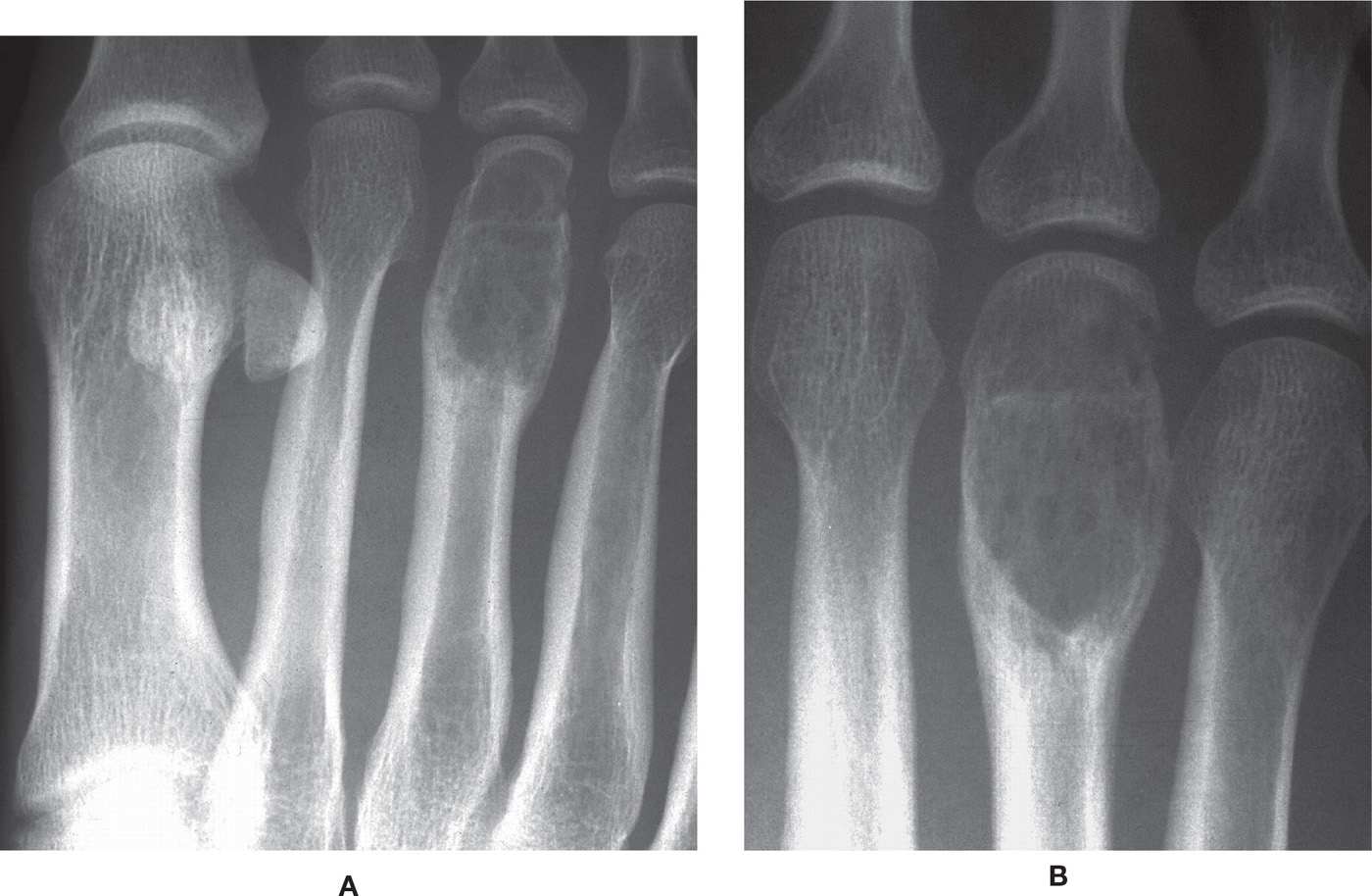
FIGURE 20-24. Giant-cell tumor, metatarsal. A 24-year-old patient presented with symptomatic lesion. A: Medial oblique view. B: DP view. An expansile, geographic type IB lesion with metaphyseal epicenter is noted in the distal third metatarsal bone. The lesion extends to the subarticular cortex but is without transarticular spread. Sparse epiphyseal septation is noted. Close-up evaluation demonstrates the presence of several smaller holes, suggesting possible moth-eaten destruction (type II destructive pattern) and therefore degeneration of a benign process. The transverse linear sclerotic zone of the proximal epiphyseal area suggests fracture. Final diagnosis was aggressive GCT.
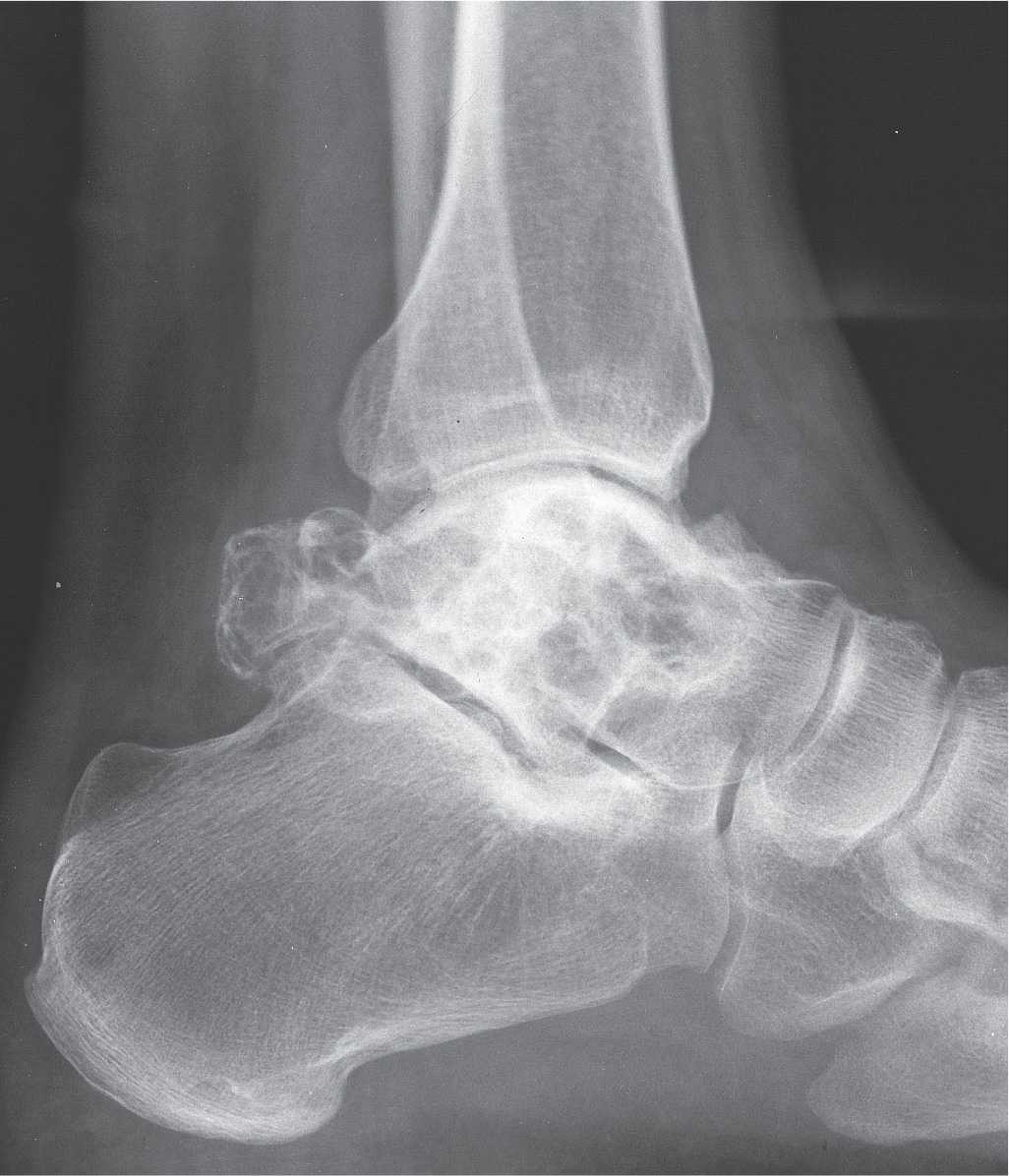
FIGURE 20-25. Giant-cell tumor, talus. Large, geographic type IA destructive lesion encompasses most of the talus. Multiple trabeculations are seen throughout the histologically confirmed lesion.
The presence of an open physis argues strongly against the diagnosis of GCT. Conversely, lesions virtually never solely occupy an epiphysis.161
There is a paucity of overlying periosteal reaction. Unlike ABC, periosteal buttressing against the lesion (“cuffs” of bone at the angle of expanded and normal cortex) is not common. As noted earlier, however, this is not to be confused with the notion that all cortical expansion can be thought of as replacement of the original cortex by a newly formed, thin periosteal shell—and cortical expansion with GCT is common. Expansile and (pseudo-) trabeculated shell formation is especially seen in the phalanges and metatarsals. This expansion can occur rapidly in 6 to 9 months, simulating other “blow-out” lesions such as ABC and desmoplastic fibroma, and involve the entire width of the bone. Pathologic fracture can occur, and complicates the diagnosis.165–167 Cortical break with soft tissue invasion may occur. There is no visible matrix seen in this lesion.
GCT is statistically (approximately 14%) the most common lesion associated with secondary ABC formation.124 In this regard, involved lesions may present with a more aggressive radiographic appearance. MRI features of the ABC components appear as typical lesions, and fluid–fluid levels may be encountered. Only the solid areas of a GCT can be biopsied to diagnose the primary lesion.
Differential Diagnosis
Differentials include ABC in the younger patient, and enchondroma in the same age range, which is generally a lobulated type IA metaphyseal lesion that lacks septation. Other epiphyseal lesions include subchondral geodes (e.g., intraosseous ganglion and intraosseous gouty tophus), chondroblastoma, occasionally CMF and infection, and rarely benign fibrous histiocytoma and clear-cell chondrosarcoma. Stacy et al.168 published an excellent pictorial differential review.
Enchondroma
Enchondroma is a benign tumor of intramedullary origin composed of mature hyaline cartilage. Bodywide, next to osteochondroma, solitary enchondroma is considered to be the second most common benign cartilaginous tumor of bone. In addition, it is considered the most common benign tumor of the hands and feet. However, its frequency is much higher (45%–60% of all enchondromas) in the hands than in the feet. The incidence of enchondroma in foot bones approximates only 7% and, therefore, is not nearly as prevalent as once thought. Other chondromas include juxtacortical/periosteal chondroma (which arise from or are adjacent to the surface of bone) and extraskeletal/soft tissue chondroma. Enchondroma outnumbers juxtacortical chondroma by more than 3:1, although in the first two decades the frequency of juxtacortical chondroma is increased.
Epidemiology
Although enchondroma is the most common tumor of the phalanges, its frequency is much higher in the hands (the hand-to-foot ratio is 6:1); of all the phalanges, it is much higher in the proximal phalanges. Therefore, the frequency of pedal enchondroma is often overemphasized. The typical age range is similar to that of GCT, which peaks in the early-to-mid–fourth decade of the 10- to 35-year-old age range. The male-to-female incidence is 1:1.169,170
Clinical Features
Enchondroma is frequently asymptomatic, but the patient may complain of nonspecific pain and swelling. Pain is often associated with pathologic fracture. Although benign in nature, sarcomatous malignant transformation may occur and should be suspected when local pain occurs in the absence of pathologic fracture.45,49 Enchondroma only rarely undergoes malignant degeneration into chondrosarcoma,171 and malignant transformation is much more common in more proximal lesions or in multiple enchondromatosis.45
Location
It is almost exclusively a tumor of the appendicular skeleton; about 50% of all enchondromas arise in the small bones of the hand, with about 20% around the knee. Most lesions have their epicenter in the metaphysis and are centrally located.
Radiographic Features (Figures 20-12, 20-26, and 20-27)
Enchondroma is classically centrally situated (“on-axis”) and is intramedullary. Its shape is round to oval and often appears as a lobulated geographic lesion with sharp margination and a thin rim of reactive sclerosis (type IA). As the lesion develops, the lobulated contour becomes more apparent. In small tubular bones, focal areas of eccentric expansile lobulation are common. Less commonly, enchondroma may manifest with a sharply defined but unreactive margin (type IB). Eccentric location in long bones is uncommon. The enchondroma typically arises within the metaphysis, although a metadiaphyseal or, less frequently, diaphyseal location may be encountered. Epiphyseal involvement is uncommon, except in the small tubular bones of the hands and feet.
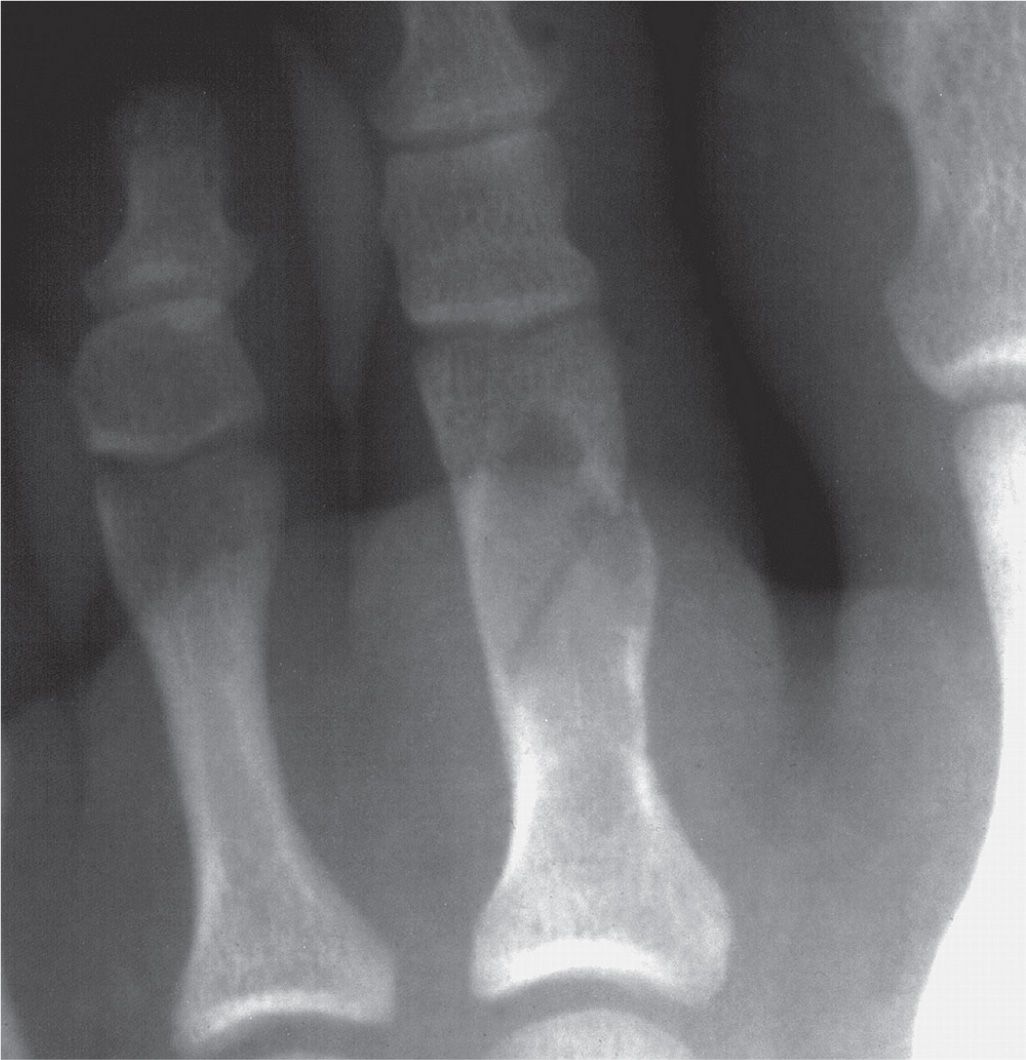
FIGURE 20-26. Enchondroma. Pathologic fracture through an enchondroma in a 25-year-old male patient. Obvious dactylitis of second digit along with the oval-shaped, lobulated lesion demonstrates geographic type IA destruction of the proximal phalanx. The lesion extends from proximal to distal metaphysis and is centrally located. Despite this on-axis location, the distal medial diaphysis demonstrates eccentric lobulated shell expansion. No matrix calcification is apparent. Benign cartilaginous lesions such as CMF should also be considered.
Shallow endosteal “scalloping” or “cupping” results from resorption along the inner cortical margin; it generally does not exceed one-third of the thickness of the cortex in long bones of the extremities. Calcific matrix formation is common; the internal densities may be flocculent, punctate, or occasionally annular (peripheral calcification of hyaline cartilage lobules). Pathologic fracture in small tubular bones is not uncommon. There is no evidence of a soft tissue mass,172 nor is periosteal reaction seen.
Low-grade chondrosarcoma may have an identical radiographic appearance, and differentiation may only be possible on the basis of clinical and histopathologic findings.49 Fortunately, malignant degeneration of a solitary lesion almost always occurs in a long tubular bone or a flat bone, rather than in small tubular bones.45 Along these lines, certain imaging features are indicative of more aggressive behavior in long or flat bones, which include cortical breakthrough, soft tissue mass, deep endosteal scalloping of the cortex (greater than two-thirds of the cortical thickness), and significant thickening of the cortex.173,174 Whereas this may apply to certain tarsal bones, nondiaphyseal cortices of the small tubular bones are often too thin to accurately gauge the depth of cortical scalloping. The association of deep endosteal scalloping of the small bones of the hands or feet does not imply malignancy inasmuch as enchondroma is more cellular and expansile in these locations.
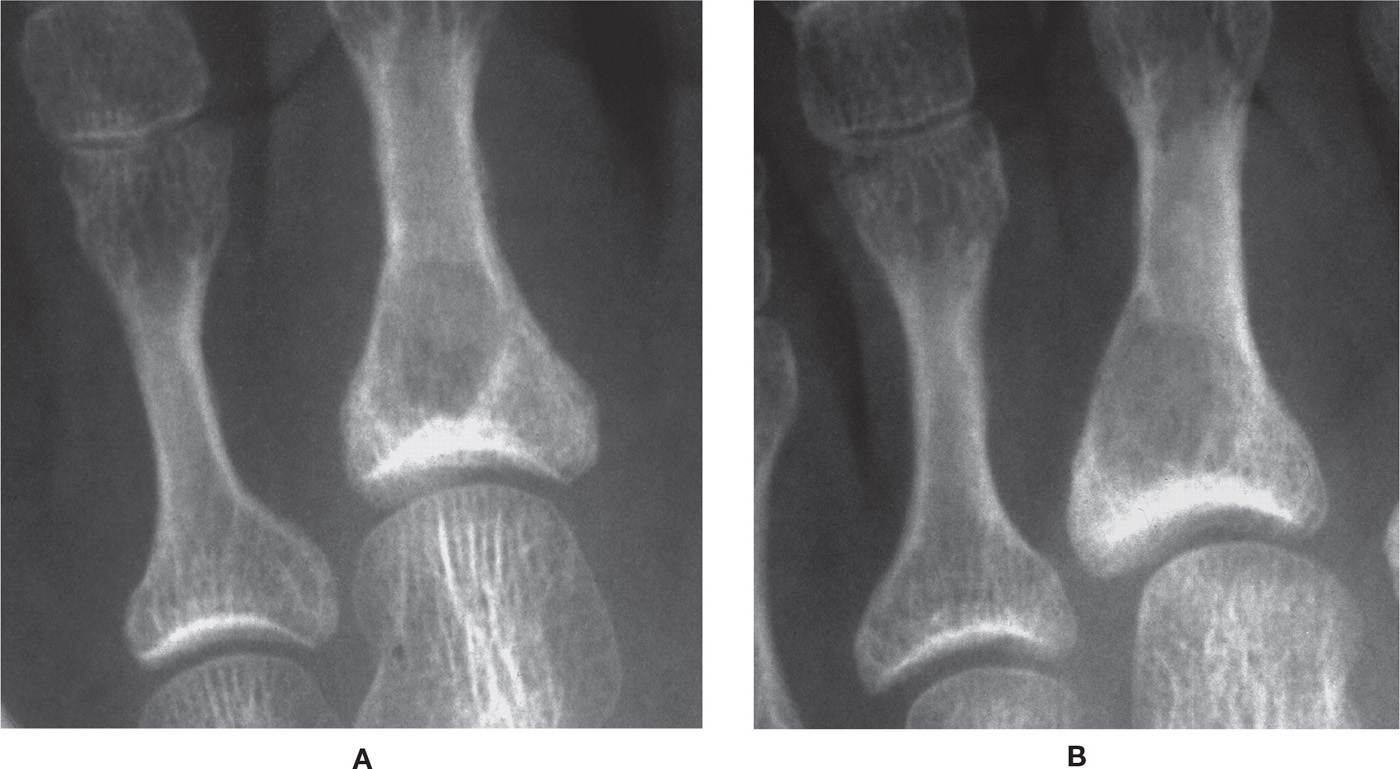
FIGURE 20-27. Enchondroma. A: Anteroposterior view. B: Medial oblique view. Smooth, round geographic type IB lytic lesion at the base of the second proximal phalanx occupies a somewhat eccentric location in a 33-year-old male patient. No septa are obvious. The biopsy-proven lesion has its epicenter in the proximal metaphysis, and the lateral cortex demonstrates mild expansion. The proximal abutment on the subarticular cortex suggests that GCT should be strongly considered. Strangely, even though GCTs are generally eccentric in origin, small tubular bone lesions may have a central predilection. In contradistinction, cartilaginous lesions of the small tubular bones may be more prone to display eccentric (off-axis) expansion at some aspect. (Courtesy of Daniel Callahan, DPM, Vandalia, OH.)
Imaging Nuances
CT is best for analyzing matrix density and cortical integrity; MRI is best for evaluating nonmineralized matrix and marrow involvement.
Differential Diagnosis
Differentials include GCT, ABC, CMF, and simple bone cyst. An enchondroma that does expand into (and occupy) the epiphysis, in the absence of internal calcification, can mimic GCT, ABC, and CMF. Calcified enchondroma in a long bone can mimic bone infarct.
Disorders of Multiple Enchondromas/Enchondromatosis
In general, these are skeletal disorders defined by the presence of ectopic cartilaginous tissue within bone. There are three disorders associated with dysregulation of chondrocyte proliferation: Ollier disease, Maffucci syndrome, and metachondromatosis.175
Ollier Disease
Ollier disease is a rare condition (1/100,000 frequency) marked by the presence of multiple enchondromas, especially involving the hands and feet, which can result in marked expansile deformities, limb-length discrepancies, and short bones. It is a nonheritable, nonfamilial dyschondroplasia, and generally presents at an earlier age (vs. solitary enchondroma). Patients with Ollier disease (and Maffucci syndrome) are at increased risk for malignant degeneration. Approximately 30% to 35% of lesions can degenerate into chondrosarcoma, and as many as one-third of patients can be affected. Malignant degeneration tends to occur in patients who are 40+ years of age.53,78,176
Clinical manifestations often appear in the first decade of life. Generally speaking, the earlier the onset of the disease, the more severe the course is. Initial presentation is frequently that of a palpable bony mass (or “knobby” swelling) on one or more fingers or toes.
Radiographically, lesions often display a somewhat larger and more expansile and aggressive appearance than solitary enchondroma. Frequently, lesions display overt cortical lysis and thickened “strands” emanating from crater-like defects. Typically, one extremity is much more affected. Chondrosarcomatous degeneration is more likely. The presence of extensive soft tissue masses should suggest Maffucci syndrome.
Maffucci Syndrome
Maffucci syndrome is a genetic, nonfamilial mesodermal dysplasia that results in multiple enchondromas and soft tissue angiomas/hemangiomas. Individuals occasionally also have lymphangioma. Radiographically, in addition to enchondromatosis, multiple phleboliths are typically encountered.177,178
Metachondromatosis
Originally described by Maroteaux179 in 1971, this disorder consists of multiple enchondromas and osteochondromas. Of the three disorders of multiple enchondromas, metachondromatosis is the only one that is inherited via autosomal dominant transmission; it appears to be secondary to a loss of function mutation in the PTPN11 gene. The disorder primarily involves the hands and feet. The osteochondromas tend to be more erratic in outline and frequently point toward the adjacent articulation rather than away from it (as is the case with most solitary osteochondromas).
Chondromyxoid Fibroma
CMF is an uncommon benign tumor characterized by lobules of myxoid and/or chondroid tissue that are separated by fibrous septa.180–182 Generally considered the least common benign tumor of cartilage, it was first described by Jaffe and Lichtenstein180 in 1948 as an entity distinctly different from chondrosarcoma. CMF is histologically characterized by lobulated areas of fibroblastic spindle-shaped or stellate cells within myxoid and chondroid tissue.180 There has been no report of metastasis.
Clinical Features
Nonspecific clinical features include mild, chronic pain and pain of long duration, which is fairly typical.183–185 Kim et al.186 reported a CMF involving the great toe distal phalanx in a 53-year-old female with an approximate 25-year duration. Soft tissue mass is occasionally seen.
Epidemiology
CMF is rare and accounts for less than 1% of all primary bone tumors. It is generally considered to be the least commonly encountered benign tumor of cartilage. The male-to-female ratio is 2:1.46 CMF is most frequent in the second to third decades; approximately 80% of patients are less than age 35. However, the occurrence of CMF in the foot may not be all that rare. According to Resnick and Kransdorf,10 collated data indicate that CMF is the fifth most common benign pedal tumor (16%), virtually tied in frequency with ABC and GCT. Seventeen percent is commonly cited for CMF involvement of the foot.187,188 However, in the Wilson et al.189 review of 38 CMFs, 8 (21% of the 38 total) lesions were of pedal origin. Overall, 15% to 31% of CMF cases have been reported to originate in the foot and ankle. Pragmatically, when radiographic features are suggestive in patients of the second to third decades, a diagnosis of CMF should be not viewed as unlikely as opposed to other benign tumors of the foot.
Location
The proximal tibia is the most frequently involved lower extremity bone in 30% to 52% of cases.181,187,176,190 The fibula and lateral ankle are occasionally involved.191,192 Although CMF is quite rare, the foot bones are involved in up to 20% of pathologically confirmed cases.193 The metatarsals are the most frequent site in the foot (6.2%–8.4%), followed by the phalanges (3.1%), the calcaneus, and the talus.181,193–197 CMF tends to be eccentrically situated in the plantar midcalcaneus when the os calcis is involved. According to Mirra et al.,198 95% of CMFs in long bones are located in the metaphysis; however, diametaphyseal and, less commonly, diaphyseal involvement is occasionally encountered in the metatarsal. In small tubular bones, metaphyseal centering with ovoid morphology is typical; however, CMF should be considered with any markedly eccentric lytic lesion occupying these areas of the bone. In long bones, CMF is not epicentered in the epiphysis, although it may extend into the epiphysis.198 (It should be noted that Park et al.199 reported a first case of juxtacortical CMF solely situated within an apophysis [lesser femoral trochanter] in a 20-year-old male.) Reports of phalangeal locations indicate centrally situated lesions, sometimes occupying the majority of the bone.186,200–203 In the latter instance, these can be quite expansile.
Fifteen cases of juxtacortical/surface CMF have been reported; the lesions did not encroach on the medullary canal. Most cases appear to involve the proximal tibia, although several cases involving the distal tibial metaphysis have been described.184,204–206 To date, none have been reported in the foot.
Radiographic Features (Figure 20-28)
CMF presents as an eccentric, geographic (oval) lesion of the metaphysis or metadiaphysis in a long bone. Lesions are likely to be round in short tubular bones. Centrally situated lesions are common in small tubular bones. Margins are well defined, surrounded by a halo of reactive sclerosis that varies in thickness (type IA). A first metatarsal CMF was discovered as an incidental finding in a 10-year-old girl presenting with juvenile hallux valgus.207 In the calcaneus, lesions tend to eccentrically involve the inferior surface of the calcaneus.
As is the case with enchondroma, the contour of CMF is often lobulated. This is most conspicuous with CT. Intralesional calcification is uncommon; when present, they are typically punctate and sparse, and are frequently identified with CT. The appearance of trabeculation is common, most likely representing the lobulated internal contours or ridges in expansile lesions.
An interrupted solid periosteal reaction (aka buttress or periosteal collarette) is occasionally encountered, primarily with eccentric lesions in long bones. However, periosteal new bone formation is generally absent in small bones of the foot.189 When detected, medullary involvement and lack of matrix calcification favors CMF versus juxtacortical chondroma.
In slender long bones such as the fibula or small tubular bones of the foot, unless discovered early, the radiographic appearance is often centrally located and may involve the entire width of the affected bone. As such, fusiform expansion with thinning of both cortices and even pathologic fracture is not uncommon,183 simulating ABC.45,184 Although the overlying cortical shell may be eroded, expanded, or thinned,194 it is frequently contained by an intact periosteum.78 Pathologic fracture is uncommon (approximately 5%–8% of cases).184
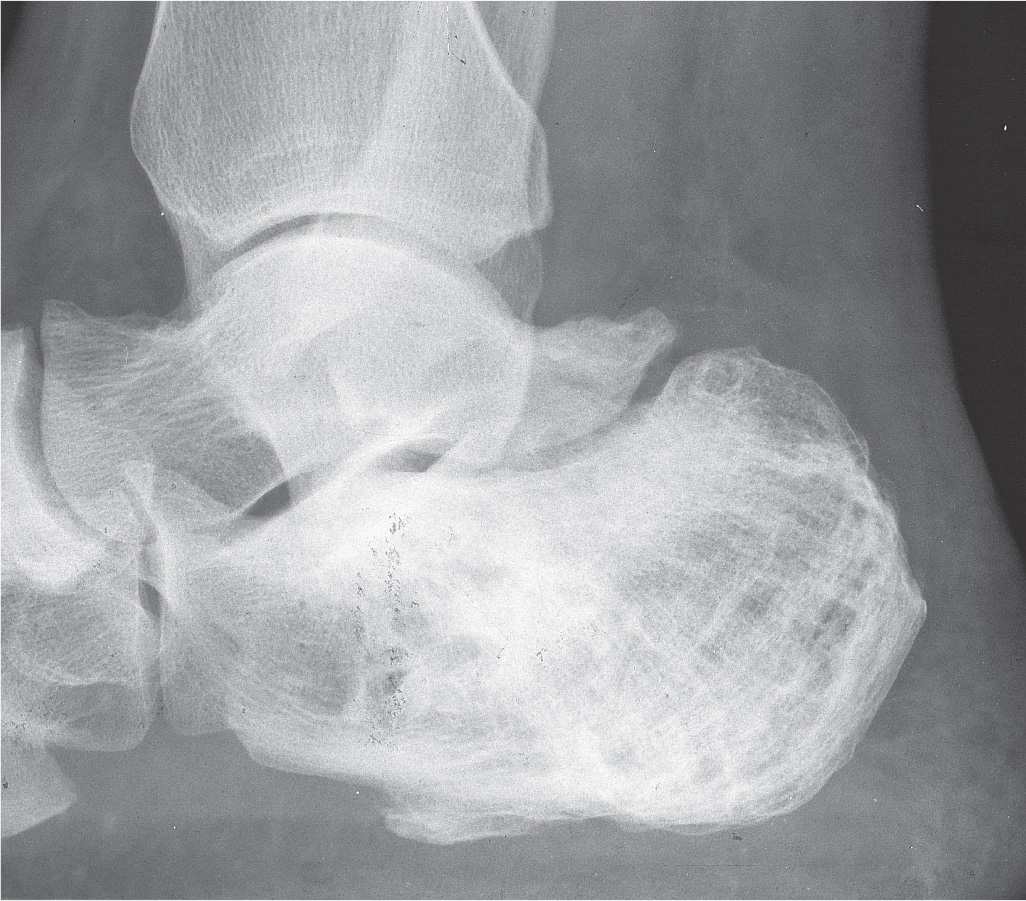
FIGURE 20-28. Chondromyxoid fibroma, calcaneus. This huge, geographic type IB destructive lesion involves the entire calcaneus except for its anterior portion. The calcaneal body is expanded, but the cortex remains intact. There are prominent, coarse trabeculations throughout this histologically confirmed lesion.
Differential Diagnosis
The more common differential diagnosis is enchondroma; which is classically central in position and often demonstrates matrix calcification. Chondroblastoma is generally epicentered in epiphyseal or apophyseal locations, and calcifications and periosteal reaction are more common. ABC presents with significant cortical expansion and frequently fractures; it also demonstrates fine trabeculations and a “soap-bubble” appearance. ABC is less well demarcated, and endosteal sclerosis is rarely observed. The solitary bone cyst is centrally located, has fusiform cortical expansion, and is frequently discovered after pathologic fracture.
Juxtacortical (Periosteal) Chondroma
Juxtacortical chondroma is an uncommon, benign neoplasm composed of mature cartilage. The lesion is largely cortical; it typically erodes the outer surface of the cortex, but does not significantly extend into the medullary cavity.
Clinical Features
The clinical presentation is nonspecific. There may be mild pain of long duration (1–5 years). Soft tissue mass is occasionally seen, appearing as dactylitis when involving the toes.
Epidemiology
The male-to-female ratio is 2:1. It most frequently appears in the first three decades.
Location
Juxtacortical chondroma has a predilection for the appendicular skeleton. The femur and humerus are the most frequent sites overall, but it may be seen in the phalanges and metatarsal bones in the foot.
Radiographic Features (Figures 20-29 and 20-30)
Radiographically, the lesion demonstrates well-defined, crater-like cortical erosion, simulating an eccentric type IA geographic (oval) lesion; its contours are smooth. The epicenter is either in the metaphysis or in the metadiaphysis. Intralesional calcification is common in about 50% of cases, which typically presents as a calcified soft tissue mass.
When a buttress periosteal reaction is detected, medullary involvement and lack of matrix calcification favors CMF. Endosteal convexity helps distinguish juxtacortical chondroma from CMF. In theory, when an eccentric lesion in an adolescent appears to be intramedullary, metaphyseal, and manifests with buttress reactions, CMF is most likely. However, anecdotally these lesions are much more likely to be located centrally when discovered in small tubular bones.
Chondroblastoma (Codman Tumor)
Chondroblastoma comprises less than 1% of all primary bone tumors and 9% of all benign bone tumors. It is an uncommon lesion, and has a predilection for epiphyseal involvement of long bones in younger patients. It is believed to arise from primitive cells of the growth plate.17 Unlike enchondroma, chondroblastoma is composed of proliferations of immature cartilage cells.
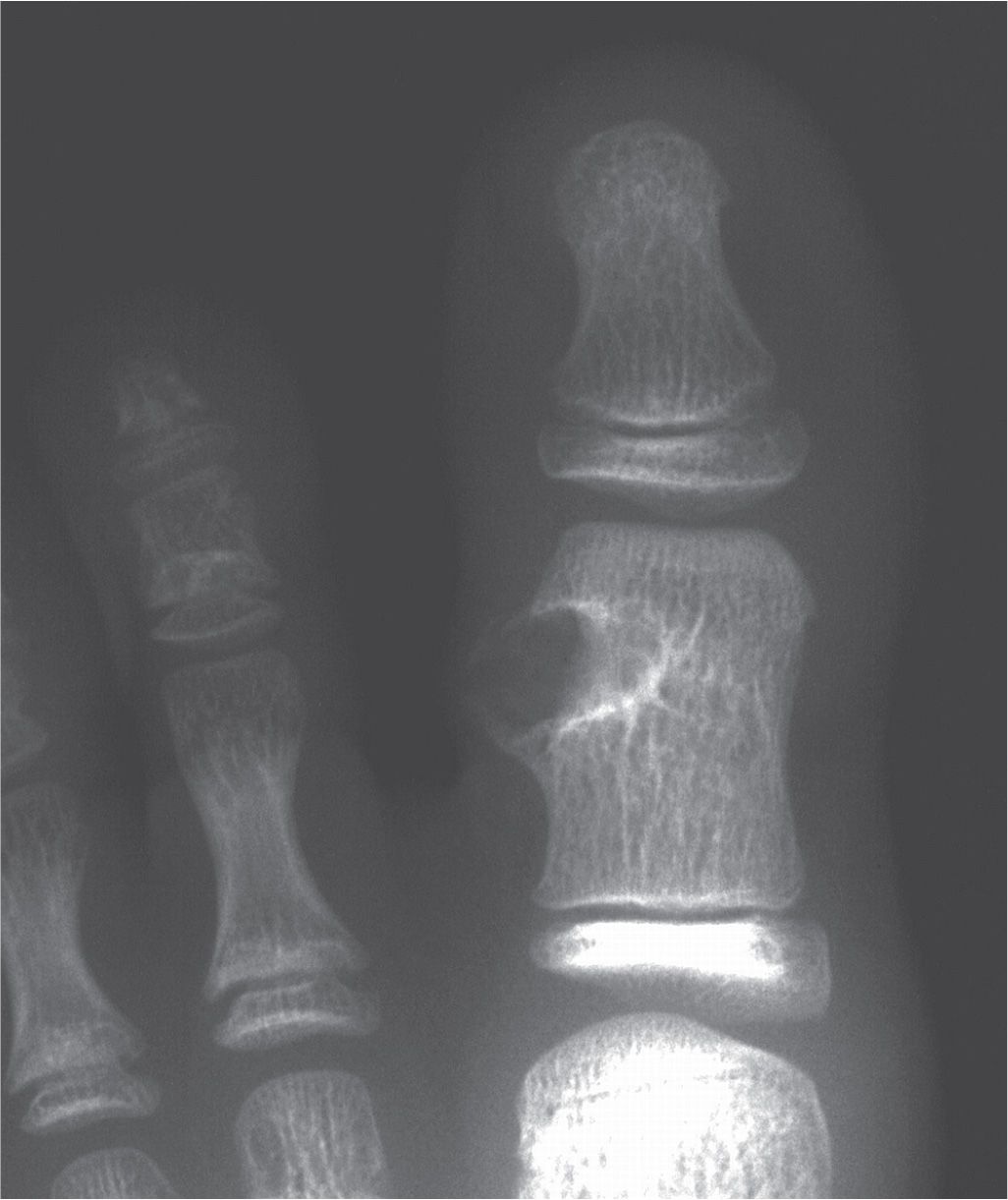
FIGURE 20-29. Juxtacortical chondroma, phalanx (9-year-old female). A shallow, cortical erosive change is centered over the distal lateral diametaphysis of the proximal hallucal phalanx in this biopsy-proven lesion. A sclerotic reactive margin is noted, with characteristic “buttress” interrupted periosteal reaction (periosteal “collarette” formation). Despite the frequent appearance of matrix calcification in these lesions, none is observed.
Epidemiology
Most patients (approximately 75%) are teenagers. Chondroblastoma is very uncommon before the age of 10 (approximately 3% of cases), and is fairly uncommon in adults over 25. Older patient ranges are, however, encountered in unusual locations like the foot.78,208–211 In general, 10% of all chondroblastomas involve the foot5;however, pedal frequency may be as high as 16%.10,211 In the Memorial Sloan Kettering study by Bakotic and Huvos,11 12 out of 82 benign foot tumors (15%) were chondroblastoma, and, excluding secondary ABC, chondroblastoma was the most commonly encountered benign foot tumor. In another large series by Fink et al.,212 pedal chondroblastoma accounted for 13% (42/322) of all lesions reviewed. Moreover,the compiled data of Resnick and Kransdorf10 indicate that chondroblastoma was the fourth most common benign foot tumor behind enchondroma, osteoid osteoma, and osteochondroma.
The male-to-female ratio is 1.4:1, although this may be higher depending on the location. Kilgore and Parrish213 found that 61% of patients with calcaneal lesions were male. In another review of 25 cases of chondroblastoma involving the hands and feet, Davila et al.210 noted a 5:1 male-to-female ratio, with all talar (8) and calcaneal (8) lesions occurring in males only.
Location
Chondroblastoma is generally found at epiphyseal sites of long bones, with a propensity for the proximal and distal femur and proximal tibia. In the foot, large series and case reports indicate that calcaneal involvement is most prevalent, followed by the talus and then metatarsal bones.46,61,143,210,211,213–233 Within the calcaneus, chondroblastoma tends to be centered eccentrically, around the peripheral articular portions (avoiding the inferior body); it is epicentered either in the apophyseal/juxta-apophyseal region or in the juxta-articular talocalcaneal or calcaneocuboid joint locations. In contrast, talar lesions have a tendency to be situated in the midbody at the time of discovery (basically abutting the ankle and talocalcaneal joints), although lesions are also discovered in the subarticular aspects of the talar head. As with most bone tumors of the foot, the cuboid, navicular, and cuneiforms are uncommonly reported pedal locations.17,78,144,234,235 A number of reported lesions in greater and lesser tarsal bones appeared cystic, and some with secondary degeneration.134,143,144,216,217,227,228,235 Multifocal chondroblastoma is rare,236,237 but there have been several reports primarily involving tarsal bones.238,239 Ohno et al.239 present a rare and fascinating case of synchronous involvement of the talus and calcaneus in a 16-year-old male.
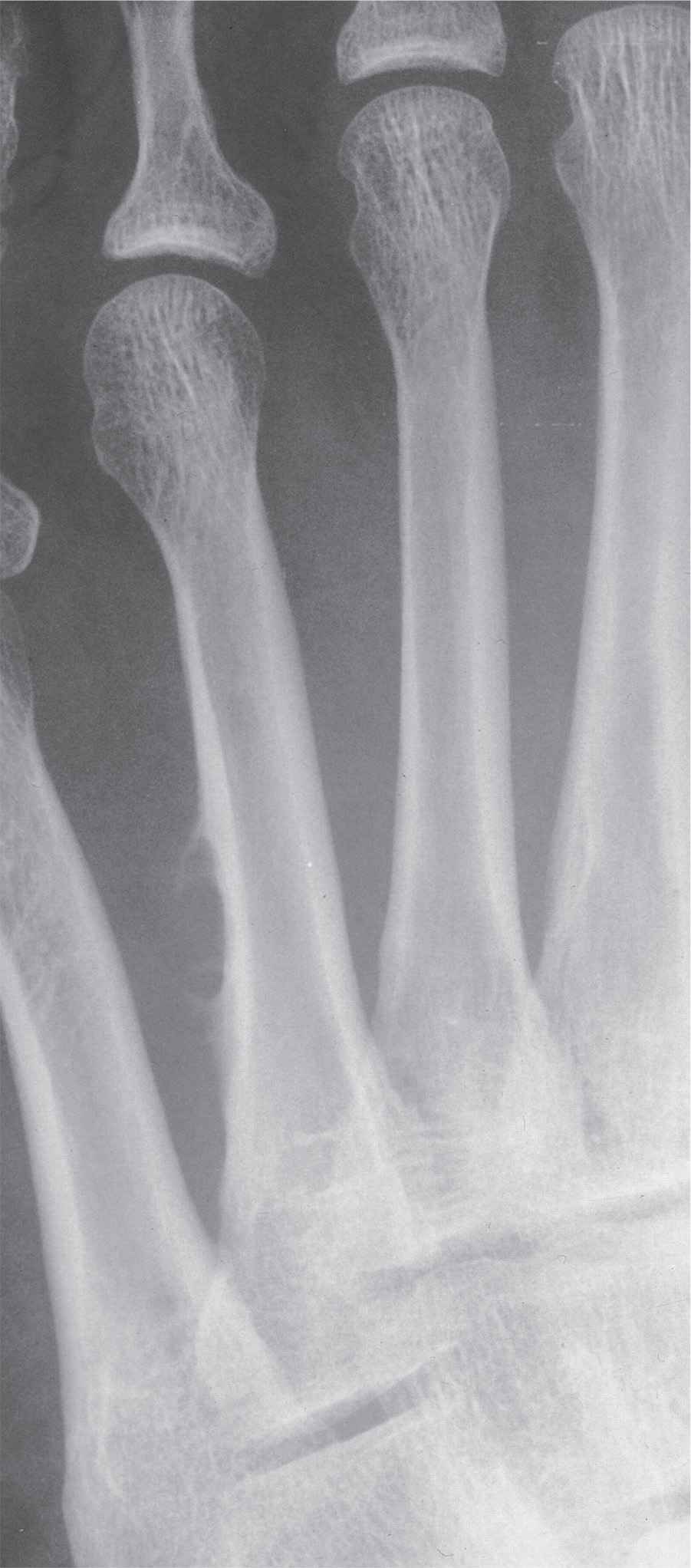
FIGURE 20-30. Juxtacortical chondroma, metatarsal. Well-defined scalloping of the periosteal surface of the cortex is demonstrated. Butress periosteal reaction is seen at its distal and proximal margins. There is no medullary involvement.
Clinical Features
Pain is a consistent initial finding, typically associated with progressive joint pain, joint effusion, and possible limitation of motion. The pain may be present for months or even years, although joint effusion is variably present.
Radiographic Features187,209,240–242
Chondroblastoma is a sharply demarcated, centrally located geographic (oval or round) lucent/lytic epiphyseal or apophyseal lesion surrounded by a rim of thin sclerotic bone (type IA). Occasionally, type IB lesions are encountered. Contours can be smooth or lobulated. Nonphyseal lesions are rarely encountered.243
Fine punctate/stippled calcifications may present in only a minority of lesions (about 7% of all pedal lesions). These subtle findings are best demonstrated on CT.
Talar lesions can be mildly expansile, whereas lesser tarsal and metatarsal chondroblastoma can demonstrate significant expansile characteristics. Not surprisingly, when chondroblastoma involves the short tubular bones of the hands and feet, even though lesions may originate in an epiphyseal location, they frequently involve most of the bone by the time of discovery.
Despite its epiphyseal location, solid or occasionally layered periosteal reactions are frequently noted adjacent to, albeit at a distance from, the primary lesion.209,244,245 In long bones, periosteal new bone formation is almost always noted in the adjacent diametaphysis.244 Surprisingly, this has also been noted in calcaneal chondroblastoma.244
Trabeculaton is variably present, but is not uncommon. Pathologic fracture is rarely reported.229 Secondary ABC is seen in up to 15% of cases and may present with mild-to-marked expansion of the cortex.
On MRI, chondroblastoma demonstrates significant surrounding marrow edema, much like osteoid osteoma. Centrally, lesions most commonly demonstrate low-to-intermediate signal on fluid-sensitive sequences.
Differential Diagnosis
ABC, GCT, clear-cell chondrosarcoma, epiphyseal enchondroma, and Brodie abscess are differentials for chondroblastoma.
Intraosseous Lipoma
Intraosseous lipoma is a benign lesion of bone comprising predominantly mature adipocytes. It is considered the most common lipogenic lesion of bone. In spite of this, intraosseous lipoma comprises 0.1% of all primary bone tumors and is considered by most to be a rare lesion.246–248 In Dahlin’s20 series, only 6 of 8452 bone tumors were intraosseous lipoma (less than 0.1%), and none of these cases involved the foot and ankle.
However, there is an ongoing debate regarding the rarity of these lesions. Some maintain that intraosseous lipoma is probably far more common than the cited 0.1%. Much of this has to do with under-reporting and/or recognition of these lesions. Inasmuch as intraosseous lipoma typically has grossly benign radiographic features, it appears as a “leave-me-alone” lesion and, consequently, there is no CT or MR follow-up. Opponents counter that many of the more recent reports of intraosseous lipoma in areas with scanty bone trabeculae, such as the proximal femoral inter- or subtrochanteric region or the calcaneal neutral triangle, are normal “pseudocystic-appearing” areas. Moreover, the calcified lipoma located in the calcaneus is secondary to an infarct and develops secondary to the rather unique vascular supply to the calcaneus.209,249,250 In a meta-analysis of 54 calcaneal lesions, Bertram et al.251 noted that intraosseous lipoma was found incidentally in 33% of the reported cases. In the authors’ experience with the foot, a number of these lesions are not followed, and even worse, when diagnosed via MR or CT by radiologists, the lesions are still not reported.
Benign osseous lipoma is now classified into three different varieties based on location: parosteal, intracortical, and intramedullary.209 The parosteal variety is exceedingly rare and comprises 0.3% of all osseous lipomas. In the foot, the vast majority of intraosseous lipomas are intramedullary, and the following discussion will focus on these lesions.
Epidemiology
Intramedullary lesions are encountered in a wide age range, from 5 to 85 years, although most are discovered in the fourth and fifth decades of life.252–254 Campbell et al.255 report a mean age of 43 years. Lesions occur infrequently in the first decade.247 There is a slight male predominance; the male-to-female ratio is 1.4 to 1.6:1.246,252,253,256(pp1480,1481)
Clinical Features
Intramedullary intraosseous lipoma is typically an incidental discovery, as most lesions are asymptomatic. Pain may occur, either insidious in onset or associated with trauma. In the former instance, pain may be secondary to increased intraosseous pressure and/or ischemia. Heel pain may be noted with activity.257 Karthik and Aarthi258 report a case of calcaneal intraosseous lipoma mimicking plantar fasciitis, and Atay et al.259 report a rare lesion of the talus presenting with an osteochondral defect. Dickson et al.260 reported a case of intraosseous lipoma of the distal tibia in which the patient related slight ankle pain for approximately 14 years prior to presentation. Milgram261 discovered seven nontender soft tissue masses in a review of 61 intraosseous lipomas, although nerve compression is a consideration when present. Pain is a general indication for surgical curettage/extirpation, as pathologic fracture is uncommon. Milgram261 reported pathologic fracture in only 1 of his 61 cases.
Location
Intraosseous lipoma can involve any portion of the skeleton, though the majority of lesions affect the lower extremity long bones or the junction of the anterior and middle third of the calcaneus (midbody/neutral triangle). In a meta-analysis of 145 cases of intraosseous lipoma by Campbell et al.,255 71% of lesions occurred in the lower extremity, with 32% in the os calcis. Approximately 20% involve the femur, 13% the tibia, and 6% the fibula. Lesions are most commonly centered in the metaphysis. There is some debate, but Mirra256 suggests that epiphyseal involvement is not uncommon, whereas involvement of the shaft is unusual. In a meta-analysis of 54 calcaneal lesions by Bertram et al.,251 33% of reported intraosseous lipomas were incidental discoveries. In fact, intraosseous lipoma affecting the foot and ankle are now recognized as being relatively more common than early demographic studies indicated. Lesions can affect the biomechanics of the ankle.262 In the foot, the os calcis is overwhelmingly involved; a number of isolated case reports affecting the neutral triangle of the calcaneus can be found in the literature, along with others as part of a larger series.255,263–282 Calcaneal intraosseous lipoma typically presents as a unilateral tumor; however, there have been reports of bilateral presentations,278–281 The authors (LO and RP) have personally encountered a bilateral case in a 28-year-old female incidentally discovered with MRI. Freiberg et al.282 reported multiple intraosseous lipomas in a patient with type IV hyperlipoproteinemia. Beyond the os calcis, there are a few reports of involvement of other pedal bones, specifically the talus and metatarsals (especially the fifth).283–286
Radiographic Features209,248,252–254,256,257,261, 287 (Figures 20-31 and 20-32)
Intramedullary intraosseous lipoma is a geographic lucent/lytic lesion with a narrow zone of transition. It has a smooth outline (lobulated contours are fairly uncommon), and is commonly surrounded by a thick or thin sclerotic rim (type IA). Occasionally, type IB lesions are encountered.
In long bones, lesions are more typically eccentric and situated in the metaphysis, metadiaphysis, or, occasionally, the epiphysis. In slender tubular bones, lesions become centrally situated over time, and mild expansion is not uncommon.
Central intralesional flocculent or ring-like calcification or ossification is noted in up to 60% of calcaneal lesions. This is sometimes referred to as the “cockade sign” (named for the identifying rosette or knots worn on late 18th century French hats) and can be pathognomonic in the midbody of the calcaneus.263,288–291 Over time, maturation of calcification leads to ossification. CT can be diagnostic, with intralesional Hounsfield units (HU) in the −60 to −100 range.254,292–294
Mild cortical expansion in long bones is sometimes seen (30%).255 Periosteal reaction is rare, and when present, is indicative of pathologic fracture.
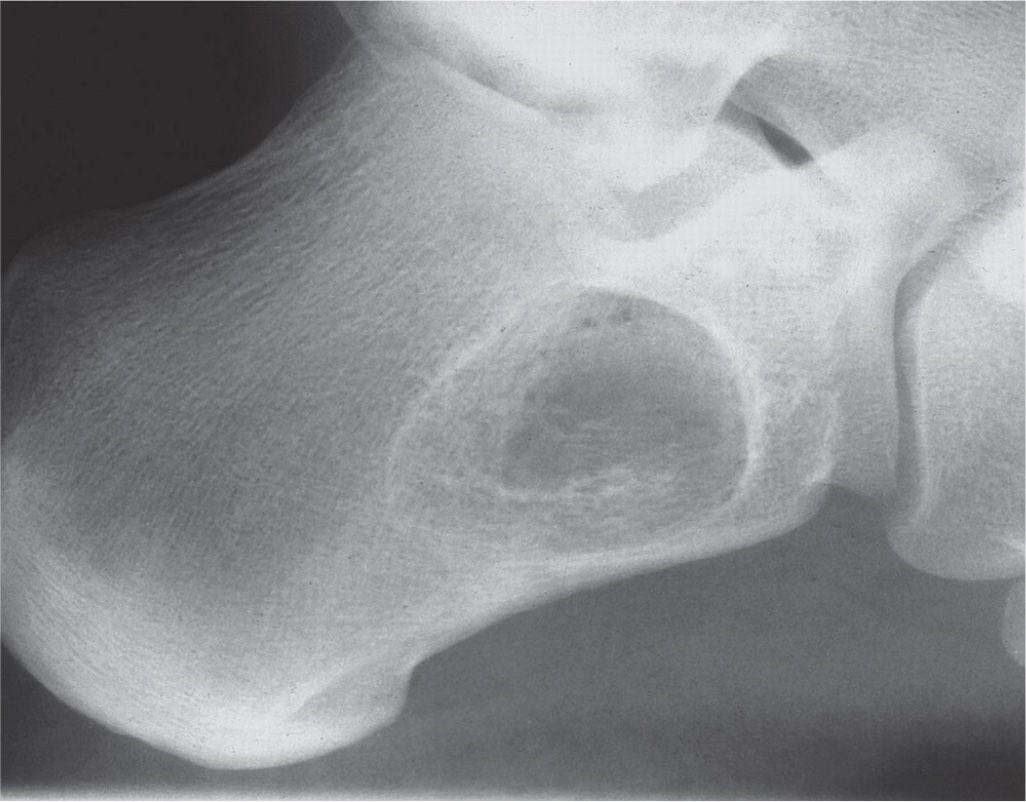
FIGURE 20-31. Intraosseous lipoma. Lateral view of this 61-year-old female demonstrates a somewhat triangular geographic IA lesion occupying the neutral triangle area of the os calcis. Faint calcification is suggested.
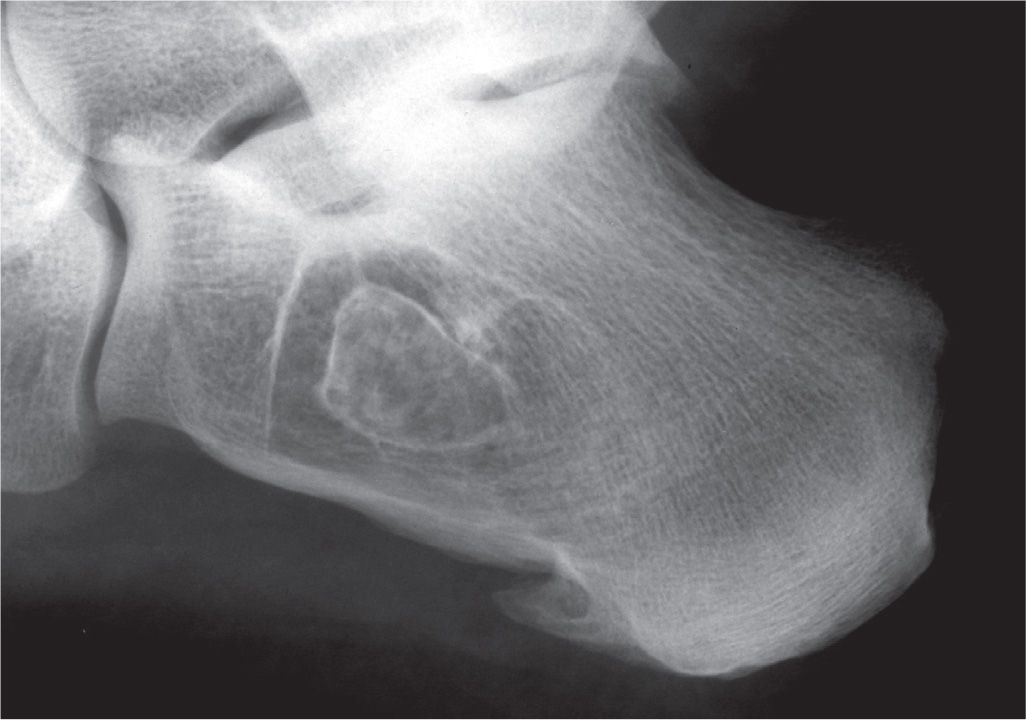
FIGURE 20-32. Intraosseous lipoma. A geographic type IA destructive pattern in the calcanel neutral triangle demonstrates a well-defined calcific rim centrally, producing a bull’s-eye appearance.
The radiographic features of intraosseous lipoma often parallel the histologic features of the lesion. Milgram261 detailed a well-known histopathologic imaging classification based on the combined features of surgical specimens from 61 cases (Table 20-6).248
Differential Diagnosis
Differentials include normal anatomy (“pseudocyst”), bone infarct, unicameral bone cyst, and ABC. Because of the common site (calcaneus) and similar clinical presentation, intraosseous lipoma may be difficult to differentiate from simple bone cyst. Simple bone cyst generally presents at an earlier age and lacks intralesional calcification or ossification, which has been reported in up to 60% of calcaneal intraosseous lipomas.255 In this regard, CT and/or MRI are usually diagnostic.
Osteoid Osteoma
As defined by the World Health Organization,246 osteoid osteoma is “a benign osteoblastic lesion characterized by its small size (usually less than 1 cm), its clearly demarcated outline, and the usual presence of a surrounding zone of reactive bone formation. Histologically, it consists of cellular, highly vascularized tissue made up of immature bone and osteoid tissue.” The small central area of active new bone formation is known as the nidus. The nidus is the active area of the lesion, and as noted earlier, it is composed of a richly vascular connective tissue that interlaces trabeculae of osteoid and calcified bone surrounded by osteoblasts. Groups of unmyelinated nerve fibers surrounded by small arterioles have been found in the center of the tumor nidus. This interposition of nerve fibers may, in part, account for the significant pain associated with active lesions. Some have proposed renaming osteoid osteoma as a circumscribed osteoblastoma, in light of its histologic similarity with osteoblastoma.295
Epidemiology
Osteoid osteoma comprises about 11% of all benign bone tumors and about 3% of all osseous neoplasia. Reports of foot and ankle involvement generally range from 3% to 15% (averaging 7.5%). Therefore, although pedal involvement is decidedly not common, it probably should not be considered rare. Osteoid osteoma occurs most frequently in the first 25 years of life; 75% occur between the ages 5 and 25 years. The male-to-female ratio is 2:1.
Location
Osteoid osteoma is generally found in the proximal femur and the tibial diaphysis. The talus (especially the neck) is the most commonly involved foot bone (along with osteoblastoma), followed by the os calcis. Two well-known sites are the junction of the dorsal talar neck with the body, and the superolateral calcaneal surface angle, also known as the crucial angle of Gissane. The frequency of talocalcaneal joint involvement parallels that of the talocrural joint.
Clinical Features
Patients typically complain of a dull, aching pain localized to the region of involvement, which is generally worse at night. Occasionally, the onset of symptoms can be correlated with antecedent trauma. Regional swelling is common, especially when the toes are involved (dactylitis). Classically, the pain is relieved by aspirin/salicylates (probably related to local prostaglandin vasodilatory activity). It is important to note that this does not always occur. Periarticular locations may present as monoarticular arthritis with pain, swelling, and limited range of motion, occasionally simulating tarsal coalition. Marked hypertrophy of the nail plate has been reported in cases that involve the distal phalanx; in this case, the differential includes osteochondroma, intraosseous glomus, inclusion cyst, and enchondroma.
| Intraosseous Lipoma: Milgram’s Histopathologic Imaging Classification261 |
Type | Histology | Diagnostic Imaging |
1 | Sharply delineated, viable fat cells with homogeneous fat content | Radiographically, resorption of trabeculae with fatty replacement |
2 | Transitional, with partial fat necrosis, and areas central or peripheral calcification or ossification | Radiographically, development of sclerotic perilesional shell (or neocortex) with calcification of necrotic fat |
3 | Largely necrotic fat, calcification of necrotic fat, fibrous proliferation, variable degrees of cyst formation, and reactive woven bone formation | Difficult to discern radiographically MRI classically demonstrates a thin peripheral rim of fat with central calcification/ossification. The radiographic thick rim of surrounding sclerosis has low-signal intensity on T1- and T2-weighted sequences. Areas of fat necrosis have a variable signal on T1-weighted and increased signal on T2-weighted sequences. Sometimes the outer zone can be composed of fibro-fatty tissue that appears heterogeneous in signal. The overall appearance has been likened to that of a “bull’s eye”254 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








