There is little argument that diabetes and its complications affecting the foot have become a major public health challenge in the Western and emerging worlds. Despite many decades of progress in the understanding of the pathophysiology and treatment of diabetes and foot complications, both major and minor lower-extremity amputations remain an all-too-common endpoint, signifying a failure to control irreversible ischemia or opportunistic infection. The rising cost of social and economic impacts on health systems of the sequellae of diabetic foot complications continues to be well documented.1–3
The term “diabetic foot” can been defined as the spectrum of pathologies affecting the foot due to secondary complications of diabetes such as peripheral neuropathy, peripheral arterial disease (PAD), Charcot neuropathic osteoarthropathy (CN), foot ulceration, and osteomyelitis. Type 1 and type 2 diabetes mellitus cause the same foot complications. However, it is the extraordinary increase in the prevalence of metabolic syndrome leading to type 2 diabetes mellitus around the world that has driven the concurrent rise in foot complications associated with diabetic peripheral neuropathy (DPN), PAD, and CN.4,5
Unlike many other foot pathologies, diabetic foot complications are often considered time-critical, since delays in initiating correct treatment may lead to more devastating consequences. Therefore, a thorough understanding of the causal pathway issues leading to complications within the diabetic foot is critical prior to interpreting radiological examinations in these patients.6,7
SELECTING APPROPRIATE INVESTIGATIONS
Almost all forms of radiological investigation have been reported to offer useful information in assessing diabetic foot complications. Radiography, despite its limitations, is recommended as the first-line investigation for complications affecting the diabetic foot due to its wide availability, low cost, and broad usefulness.8–10 However, the limitations, sensitivity, and specificity for assessing each method within the context of the diabetic foot are critical to determining the appropriate investigation to undertake, especially in time-critical emergent infection, which may be either limb or life-threatening.
Radiography is recommended for the assessment of CN, osseous structural alignment, bone infection, and the presence of radiopaque foreign bodies or gas in soft tissues.11,12 Due to poor soft tissue definition, radiography lacks the ability to evaluate soft tissue infection such as cellulitis, abscess, or drainable collections.13 In the setting of a specialized diabetic foot unit, Aragón-Sánchez et al.14 demonstrated that when combined with a probe-to-bone test, radiography has excellent specificity and sensitivity for osteomyelitis, with only 6.6% of patients failing to show histologically confirmed osteomyelitis when assessed in this manner.
The American College of Radiology (ACR) Appropriateness Criteria for suspected osteomyelitis of the foot in patients with diabetes mellitus is a useful adjunct to decision making for imaging the diabetic foot (Table 22-1).8
Magnetic resonance imaging (MRI) has increasingly played a more prominent role in the assessment of diabetic foot complications, due to its fundamental ability to evaluate both soft tissue anatomy and bone tissue in the one examination. This is particularly useful when evaluating mixed osseous and soft tissue infection. However, where contraindications to MRI exist, or it is unavailable, CT, SPECT/CT, and nuclear medicine investigations continue to provide useful adjunctive information about diabetic foot pathologies.
GENERAL CONSIDERATIONS IN THE DIABETIC FOOT
Nonspecific Periosteal Reaction
Periosteal reaction is a typical finding in early osteomyelitis and cortical stress injury. However, nonspecific periosteal reaction (unrelated to infection) can also occur in neuropathic feet.
Cavanagh et al.11 undertook a widespread review of radiographs for diabetic foot assessment and noted a high incidence of nonspecific periosteal reaction in the feet of neuropathic patients, unrelated to infection. This confirmed the findings of Williams et al.,15 who postulated that in the absence of any skin lesions, these nonspecific periosteal reactions were the result of bending stress in long bones related to the well-recognized elevated mechanical stress in the neuropathic foot.
Biomechanics and Peripheral Neuropathy
Much evidence exists to support the premise that altered biomechanics plays an important role in the pathogenesis of neuropathic ulceration and CN.16–18 An appreciation of these influences can assist in better understanding the etiology of neuropathic lesions, along with the prognosis for the partially amputated foot and the emergence of neuroarthropathy.
The role of nonenzymatic glycosylation of collagen in causing generalized limited joint mobility and diabetic cheiroarthropathy, with associated contracture of the posterior calf muscle group, is thought to be at least partially responsible for increased plantar pressures under the forefoot in patients with diabetes.17,19 Studies on the effectiveness of tendo-Achilles lengthening as a curative surgical measure for chronic or recurrent plantar forefoot ulceration appear to support this view.20–22
| Choosing Appropriate Imaging Investigations according to Variants in the Clinical Presentation in the Diabetic Foot |
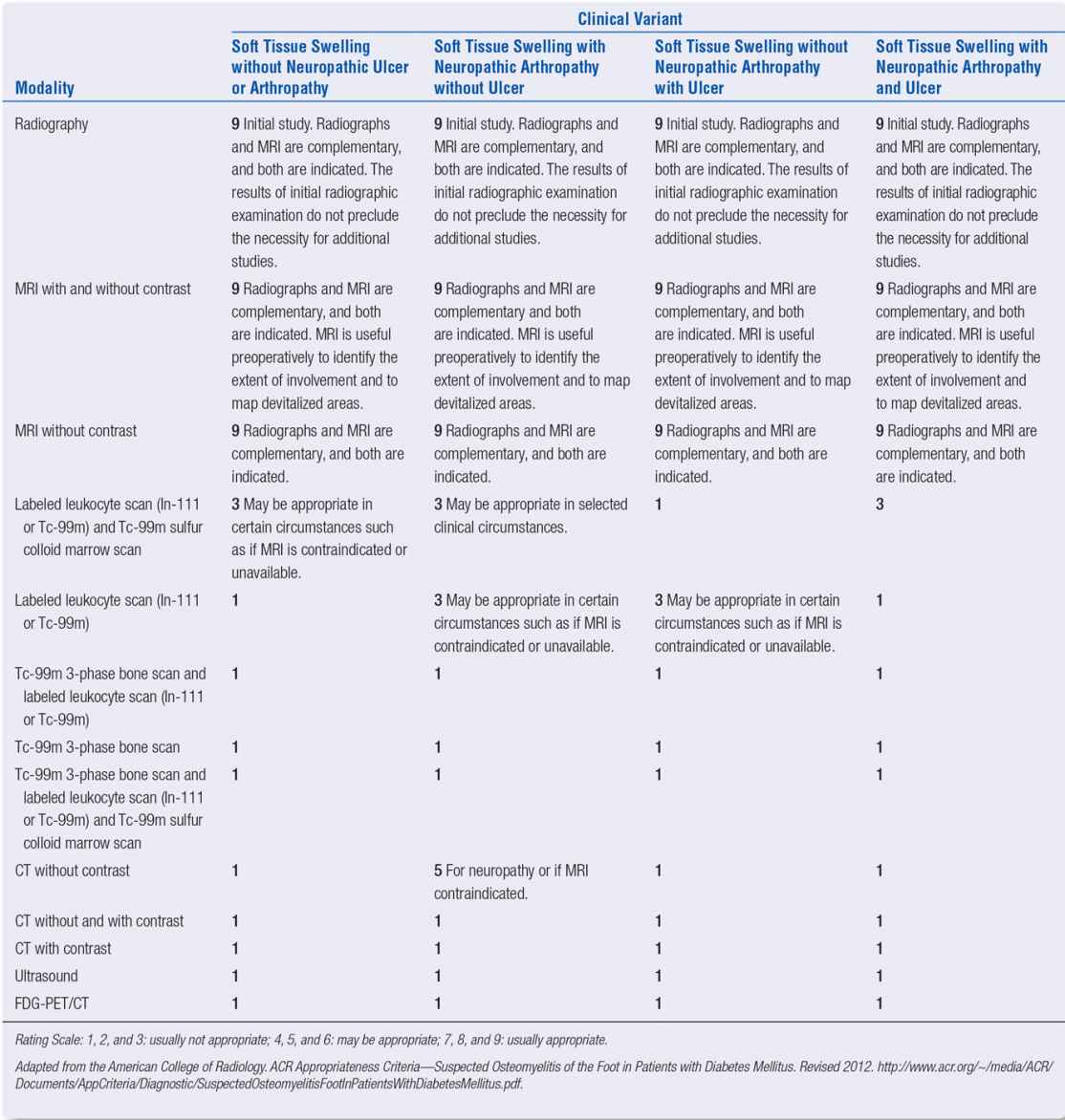
Although the length-dependent somatic component of diabetic peripheral neuropathy cannot be evaluated through medical imaging, the influence of more advanced motor changes can adversely influence the mechanical function of the foot and can be appreciated through secondary evaluation of the foot via MRI (Figure 22-1). Newer techniques in MR neurography have shown promise in terms of identifying focal entrapment mononeuropathies on larger nerve more proximal to the foot and ankle.23
MRI studies of the neuropathic foot in diabetes have also shed considerable information on the role of intrinsic muscle wastage and the development of digital deformity causing an intrinsic minus foot type.24 With the progression of MTP joint buckling and extensor contracture in the neuropathic foot, it is often easy to appreciate the distal migration of the plantar adipose tissue normally resident under the metatarsal heads (Figure 22-2).
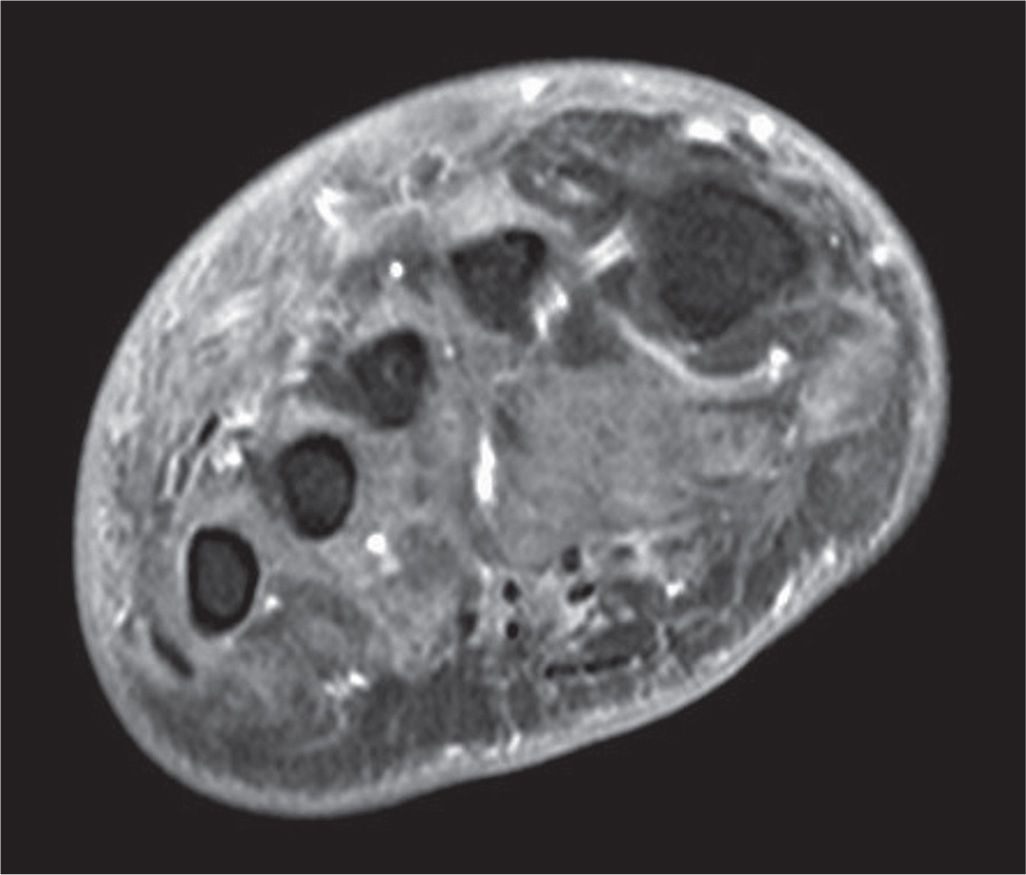
FIGURE 22-1. T1-weighted fat-saturated postgadolinium coronal MRI at the level of the metatarsal diaphyses in a patient with extensive intrinsic muscle wasting due to peripheral neuropathy. Note that the normal dark gray musculature in the central compartment has been replaced with fatty infiltration of intermediate signal. Dorsal edema and dermal thickening are also present.
Mönckeberg Arteriosclerosis (Medial Calcific Sclerosis)
In 1903, Johann Georg Mönckeberg25 first observed a process of calcification of small- and medium-sized arteries in the upper and lower limbs. His findings suggested that calcification histologically affected the media of these arteries, though recent findings suggest this may also extend to the internal elastic lamina.26,27
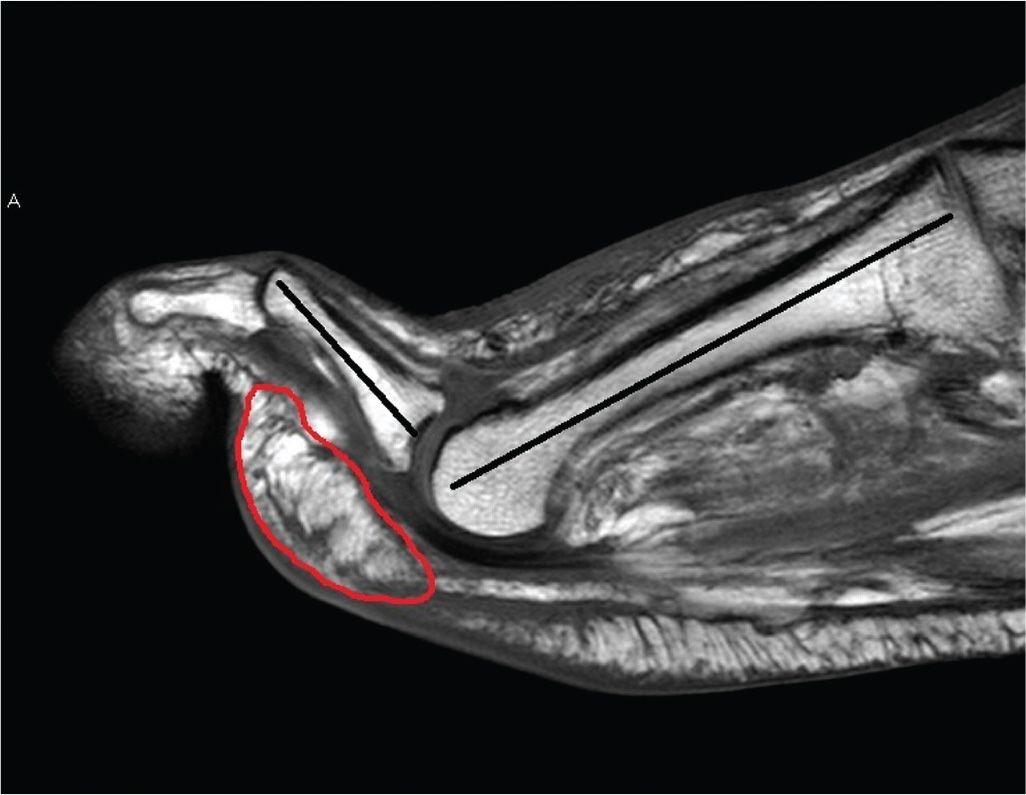
FIGURE 22-2. T1-weighted sagittal MRI showing second hammertoe deformity and secondary anterior fat pad migration (red outline) in a patient with motor neuropathy. In this scenario, there is a loss of protective adipose tissue directly underneath the metatarsal head. In the context of peripheral neuropathy, posterior equinus, and increased plantar pressure and shear stresses, this leads to increased risk of ulceration underneath the unprotected metatarsal head.
Medial calcific sclerosis is a common finding in chronic inflammatory conditions, diabetes mellitus, chronic kidney disease (CKD), and end-stage renal failure (ESRF). In the diabetic foot, it is a frequent finding with increasing duration of diabetes and is often the cause of elevated ankle-brachial pressure indices above 1.3 because of its effects on reducing the compressibility of arteries (Figure 22-3).6 However, the clinical significance of medial calcific sclerosis remains elusive and uncertain.
DIABETIC FOOT INFECTION
Despite some lingering misconceptions in the broader medical community, infection is not a cause of foot ulceration in diabetes, rather it is a secondary complication most commonly associated with neuropathic or ischemic ulceration, unrecognized foreign body penetration, or skin puncture.
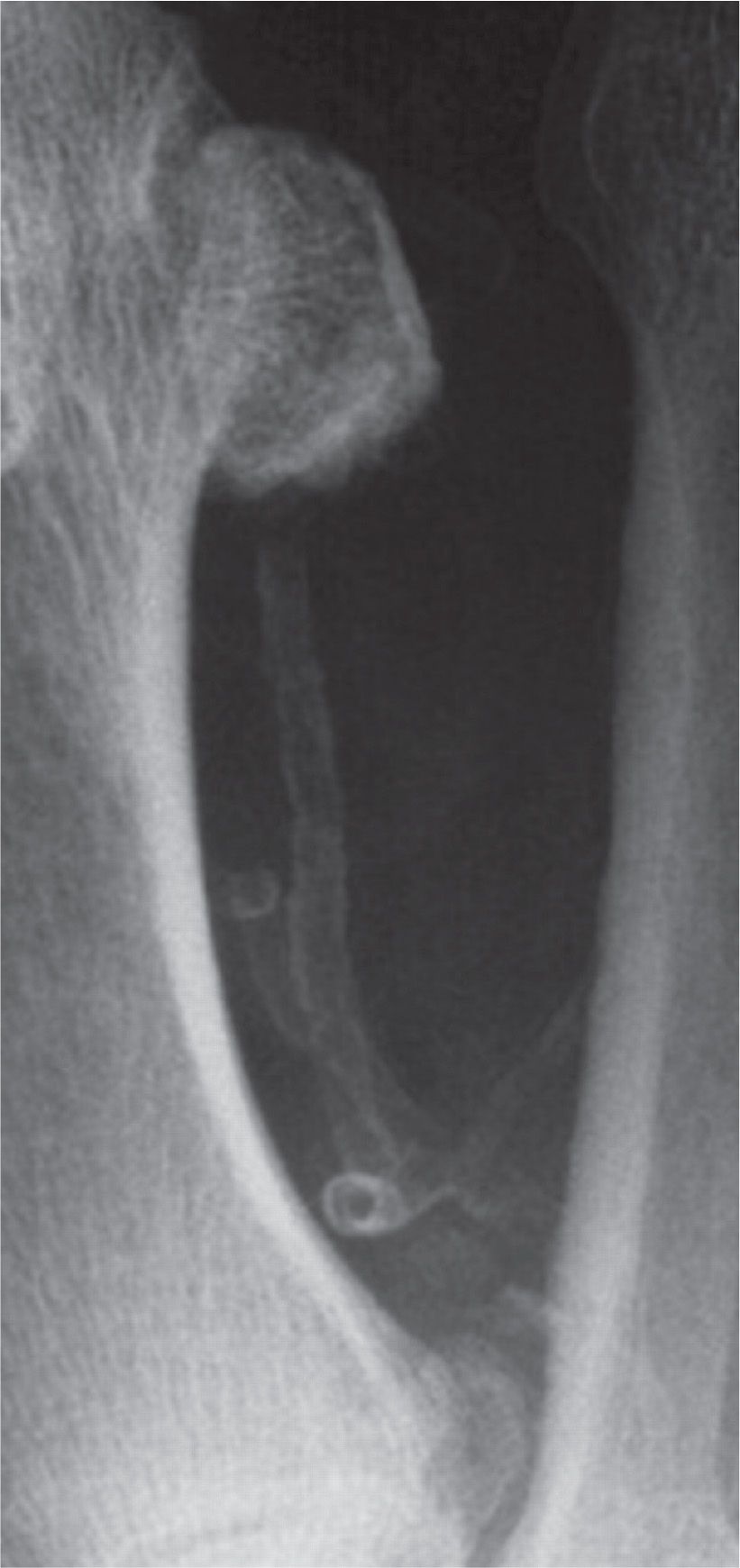
FIGURE 22-3. Dorsoplantar radiograph showing Mönckeberg sclerosis of the first plantar metatarsal artery, with the calcified lumen of the dorsal perforating branch visible in cross section.
While all forms of medical imaging may play a role in the evaluation of the diabetic foot for infection, the Infectious Diseases Society of North America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections suggests the following recommendations, based on expert consensus evaluation of the available evidence9 (Table 22-2). These recommendations, along with the ACR Appropriateness Criteria8 for suspected osteomyelitis in the diabetic foot, are consistent in the view that radiography remains, at this time, the mainstay of early initial evaluation of diabetic foot infection, with MRI the most important second-line investigation.
Soft Tissue Infection
Cellulitis, abscess, and sinus tract formation are all common findings within the clinically infected diabetic foot and can usually be appreciated visually and via careful wound examination and exploration. However, sinus tracts to deeper tissue layers may still be present, along with communication to other compartments and structures through fascial planes and tendon sheaths (Figure 22-4).6 Although ultrasound (US) examination may yield some useful information about the nature of any drainable collections in the soft tissues, MRI will be the most appropriate choice for detailed examination of the soft tissues in suspected diabetic foot infection.6,8–10 Increased signal in subcutaneous tissue layers in T2, STIR (short-tau inversion recovery), and contrast studies, especially when associated with skin ulceration, is indicative of soft tissue infection (Figures 22-5 and 22-6).
Radiopaque foreign bodies, which may directly inoculate the foot with pathogenic bacteria and cause soft tissue infection and abscess, can occur easily in the neuropathic foot. Radiography will normally allow visualization of these materials; however, multiple views will be necessary to correctly orientate position if removal is required.
Gas Gangrene
Where the infectious pathogen is exotoxin-producing soil-borne anaerobes, gas gangrene and myonecrosis may occur. Organisms such as Clostridium perfringens, Bacteroides, and less commonly Klebsiella pneumoniae are typically responsible for these potentially severe and limb-threatening infections.28,29 During the process of myonecrosis, gas bubbles may form as tissue destruction runs along fascial planes. This gas is often easily appreciated within the soft tissues radiographically as pockets of decreased density within the usual soft tissue planes (Figure 22-7), although MRI will further define any tracking of infection along fascial planes and tendon sheaths (Figure 22-8).10,13
| Infectious Diseases Society of North America Clinical Practice Guidelines for the Diagnosis and Treatment of Diabetic Foot Infectionsa |
25. We recommend that all patients presenting with a new diabetic foot infection have plain radiographs of the affected foot to look for bony abnormalities (deformity, destruction) as well as for soft tissue gas and radio-opaque foreign bodies (strong, moderate).
26. We recommend using magnetic resonance imaging (MRI) as the study of choice for patients who require further (i.e., more sensitive or specific) imaging, particularly when soft tissue abscess is suspected or the diagnosis of osteomyelitis remains uncertain (strong, moderate).
27. When MRI is unavailable or contraindicated, clinicians might consider the combination of a radionuclide bone scan and a labeled white blood cell scan as the best alternative (weak, low).
aExtract from Lipsky BJ, Berendt BR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):132–173.
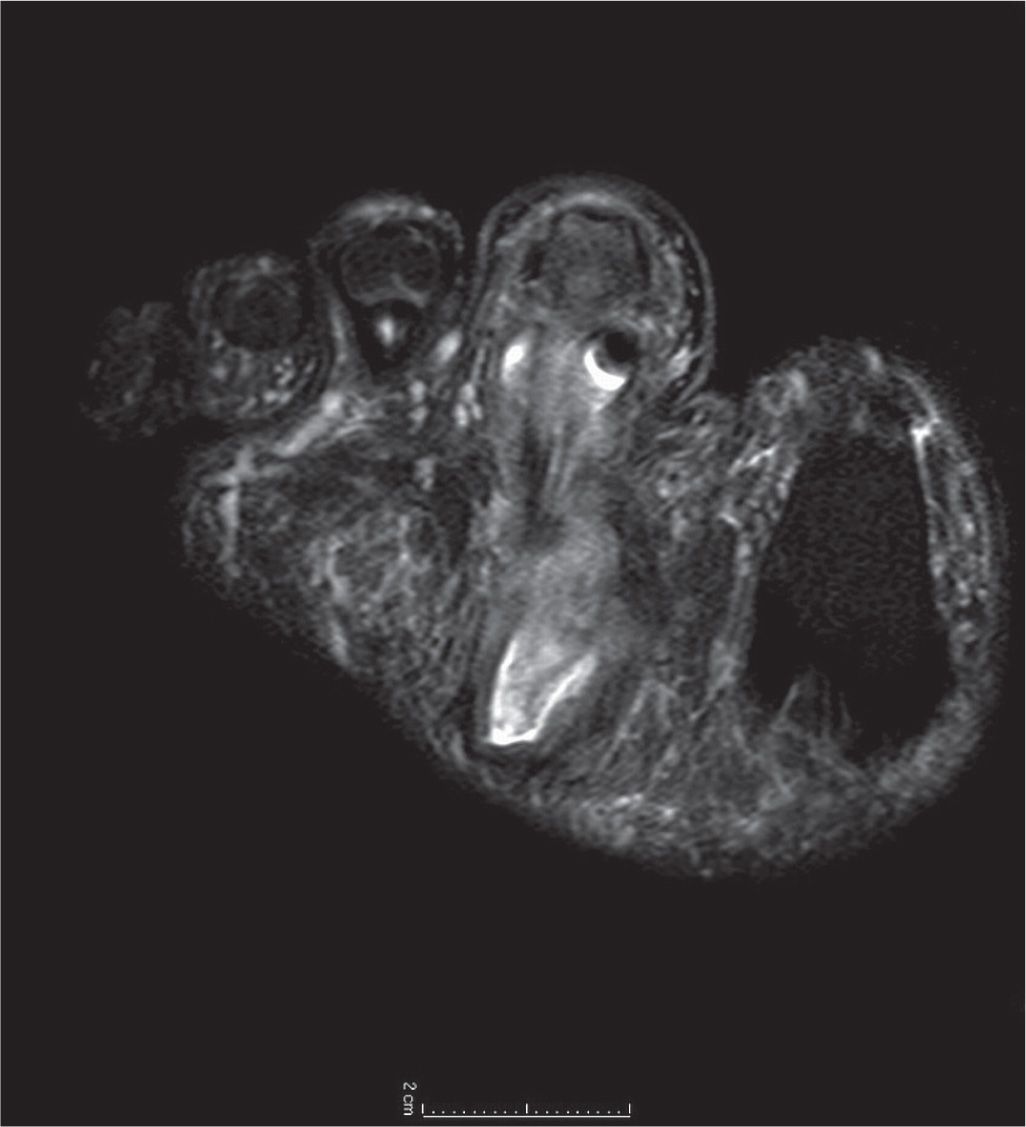
FIGURE 22-4. T2-weighted coronal MRI at the level of the base of the second toe in a diabetic patient. Note increased signal within the sheath of the flexor digitorum longus tendon, suggestive of tracking infectious tenosynovitis, along with intermediate signal more plantarly communicating to a drainable collection near the plantar aspect of the foot.
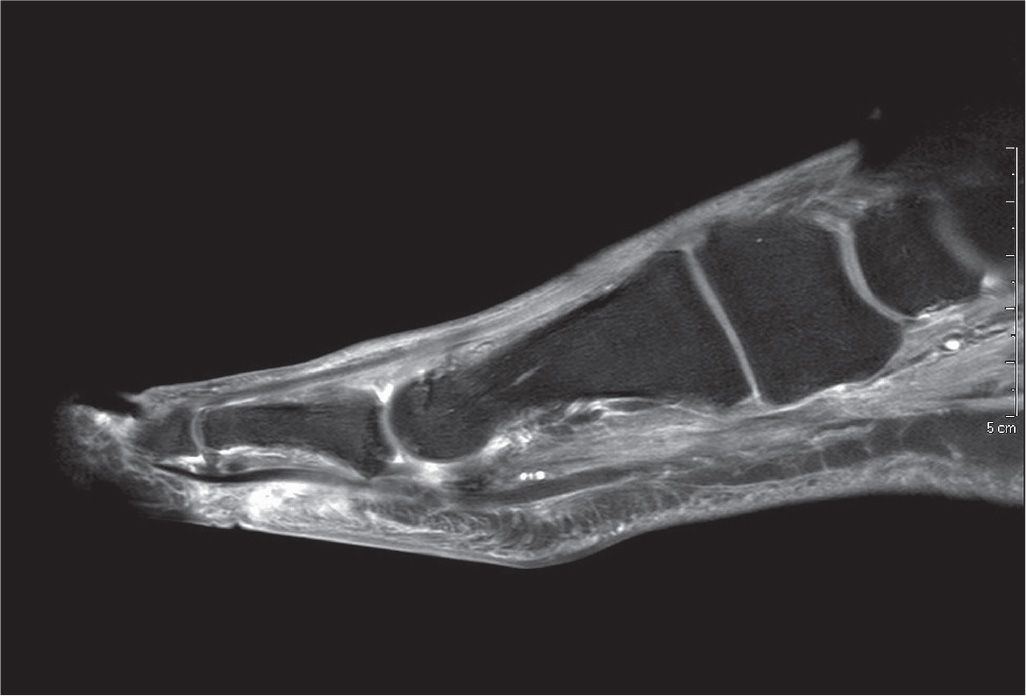
FIGURE 22-5. T1-weighted fat-saturated postgadolinium sagittal image of the first ray. Note markedly increased signal and enhancement of the soft tissue plantar to the proximal phalanx, suggestive of subcutaneous infection without abscess, or involvement of the flexor hallux longus tendon.
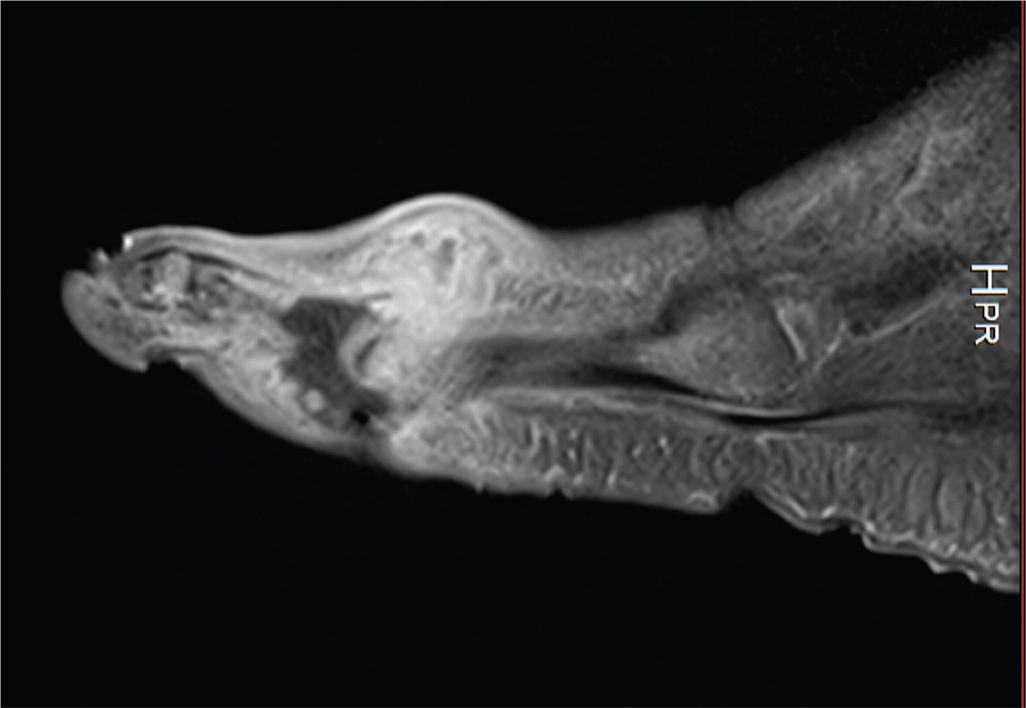
FIGURE 22-6. T1-weighted fat-saturated gadolinium contrast image of neuropathic ulceration and soft tissue abscess plantar-lateral to the fifth metatarsophalangeal joint in a patient with diabetes. Note increased signal and soft tissue distension dorsally, representing subcutaneous abscess communicating with associated plantar ulcerative defect.
Bone and Joint
Osteomyelitis and septic arthritis are perhaps the most feared diagnoses for the diabetic foot. Direct extension and contamination of bone tissue through ulcer sinus, rather than hematogenous spread, is the most common causal pathway to bone and joint infection in patients with diabetes. Commonly, neuropathic ulceration occurs under metatarsal heads, or overlying digital deformities and other bony prominences as the portal of entry for bacterial contamination. Subsequent direct extension contamination of deep fascia, joint structures and eventually bone is then easily possible if there has been no early intervention. Where exposed bone is present in a wound or can be probed directly through the wound, osteomyelitis may be considered a possibility, though a negative probe-to-bone test has much greater ability to predict the absence of osteomyelitis.30,31
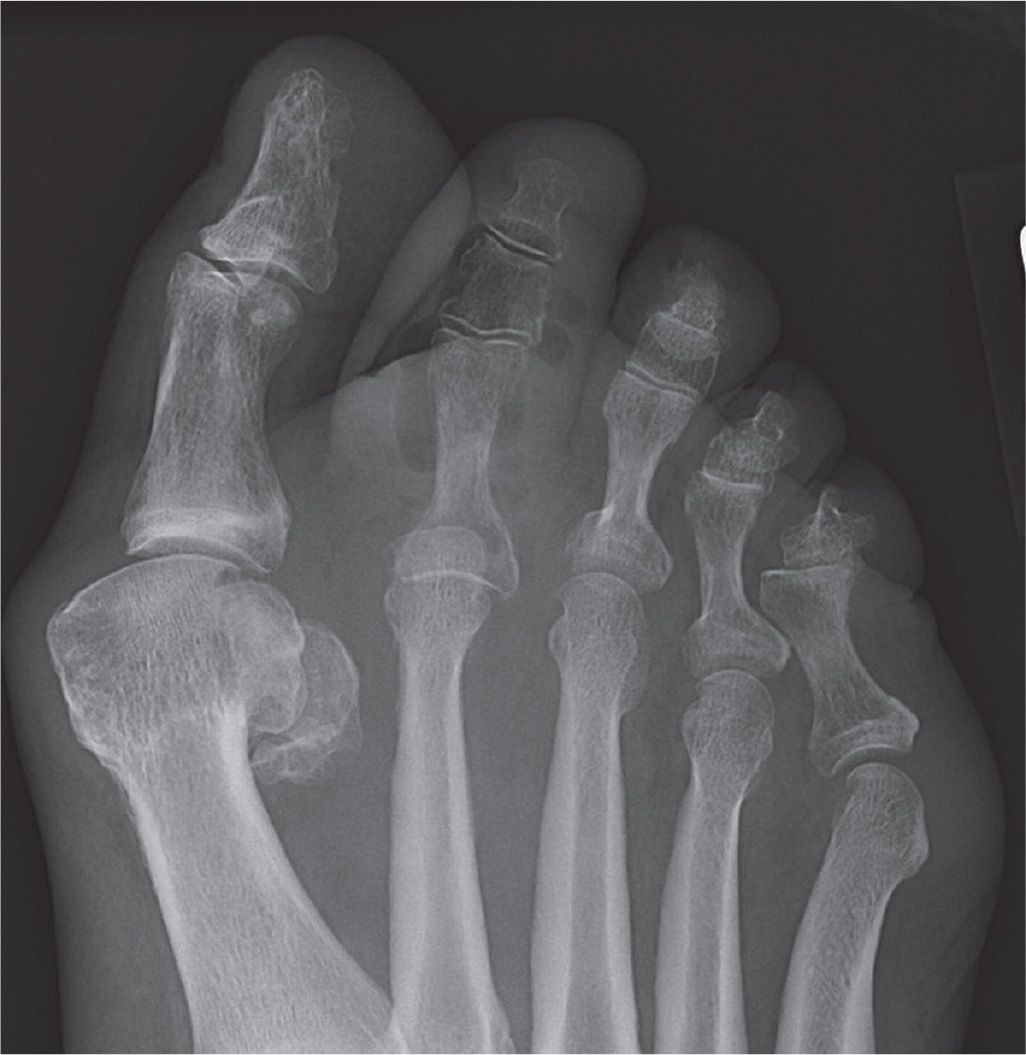
FIGURE 22-7. Early gas gangrene affecting the second toe with associated dislocation of the second metatarsophalangeal joint. Note gas bubble formation and increased soft tissue swelling in the second toe.
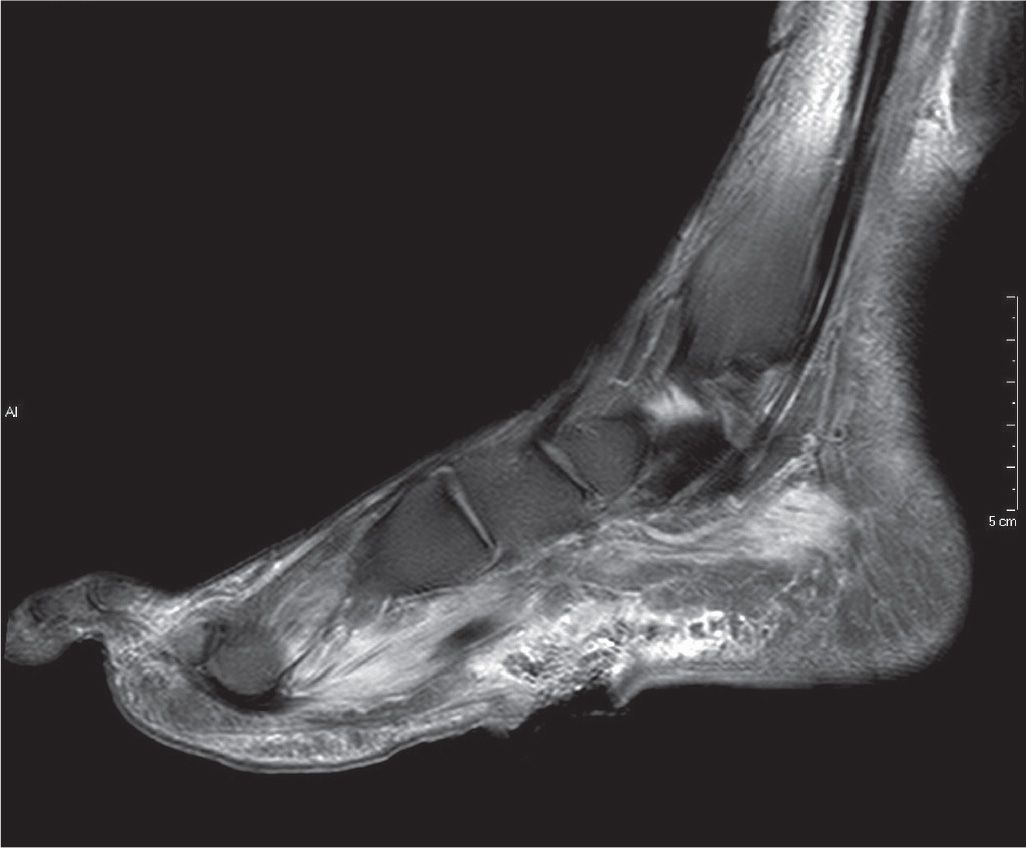
FIGURE 22-8. T2-weighted fat-saturated sagittal MRI of large (3 cm) ulceration on the plantar-medial aspect of the foot extending through the plantar fascia. Deep to this, there is extensive inflammatory infiltrate with edema throughout the plantar muscle compartments of the foot with areas of complex fluid signal at the deep surface of the ulcer, and small adjacent foci of gas.
Direct inoculation of periosteum and cortical bone via puncture wounds and foreign body penetration must also be considered with the insensate foot, where negligible evidence of skin breach may be present, and the injury may have occurred sometime in the past.13
If bacteria penetrate the periosteum, infectious periostitis may develop, signified by periosteal reaction radiographically. Progressive extension of periosteal elevation along the shaft of long bones such as metatarsals and phalanges may be easily visualized. Once the periosteum is breached, then it is likely that cortical involvement will occur leading to true osteomyelitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








