MARIE WILLIAMS AND ROBERT A. CHRISTMAN
The radiographic presentation of infection depends on several factors, including the age of patient, route of infection, type of organism involved, anatomic location, and nature of the disease process. Early diagnosis of infection, especially involving bone, allows appropriate treatment and diminishes the risk of long-term sequelae and complications.
To thoroughly understand the etiology of bone infection, the clinician must first have a basic understanding of bone pathophysiology and how infecting organisms invade bone and joints. When the vascular supply to bone is disrupted, physiologic changes take place within the Haversian and Volkmann systems affecting osteoblastic and osteoclastic function, which are responsible for normal bone function. Correlating clinical findings with radiographic findings can give the practitioner a greater comprehension of the disease process.
DEFINITION OF TERMS
Infectious periostitis is the term used for infection that invades the periosteum only and does not involve the cortex and bone marrow. With infectious (or suppurative) periostitis the changes are subtle and may be identified by a periosteal reaction (Figure 18-1). As the infection penetrates into the cortex but does not invade medullary bone, the term infectious osteitis is used (Figure 18-2). Once the infection involves both cortex and bone marrow, the more accurate term is osteomyelitis (Figure 18-3).1
It can be difficult to differentiate infectious osteitis from osteomyelitis radiographically, because a lag time of approximately 10–14 days separates the clinical presentation from the visible radiographic findings.1–3 That it because a reduction of bone density between 30% and 50% must occur before radiographic evidence is noted.1,3–6 For this reason, bone scintigraphy has been valuable in early diagnosis of osteomyelitis if no other underlying pathology is present.
Radiographically, the fascial planes of the plantar and dorsal midfoot and tarsus normally can be identified in the lateral view. As soft tissues become infiltrated with an infecting organism, the fascial planes disappear, which may be the initial radiographic sign of impending bone infection and should alert the practitioner to institute early treatment. Occasionally gas or air is seen in the affected area (Figures 18-4). Soft tissue infection of the digits appears radiographically as an increased soft tissue density and volume (Figure 18-5), which, unfortunately, is not specific for infection. If inappropriately treated or left untreated, however, soft tissue infection can lead to bone and/or joint infection.1–3,5,6
The term septic arthritis is used to describe infection of a joint. Joint infection erodes cartilage and decreases joint mobility.1,7 Radiographically, early septic arthritis appears similar to acute gouty arthritis, that is, significant increase in soft tissue volume and density with or without joint space widening or juxta-articular rarefaction (Figure 18-6). However, infection leads quickly to subchondral resorption and osteolysis. Septic arthritis can later lead to bony ankylosis (Figure 18-7).
CLASSIFICATIONS OF OSTEOMYELITIS
Osteomyelitis has been classified according to several methods: its clinical presentation and onset, the transmission route of the infecting organism, patient age, its anatomic type, and the infecting organism.8,9
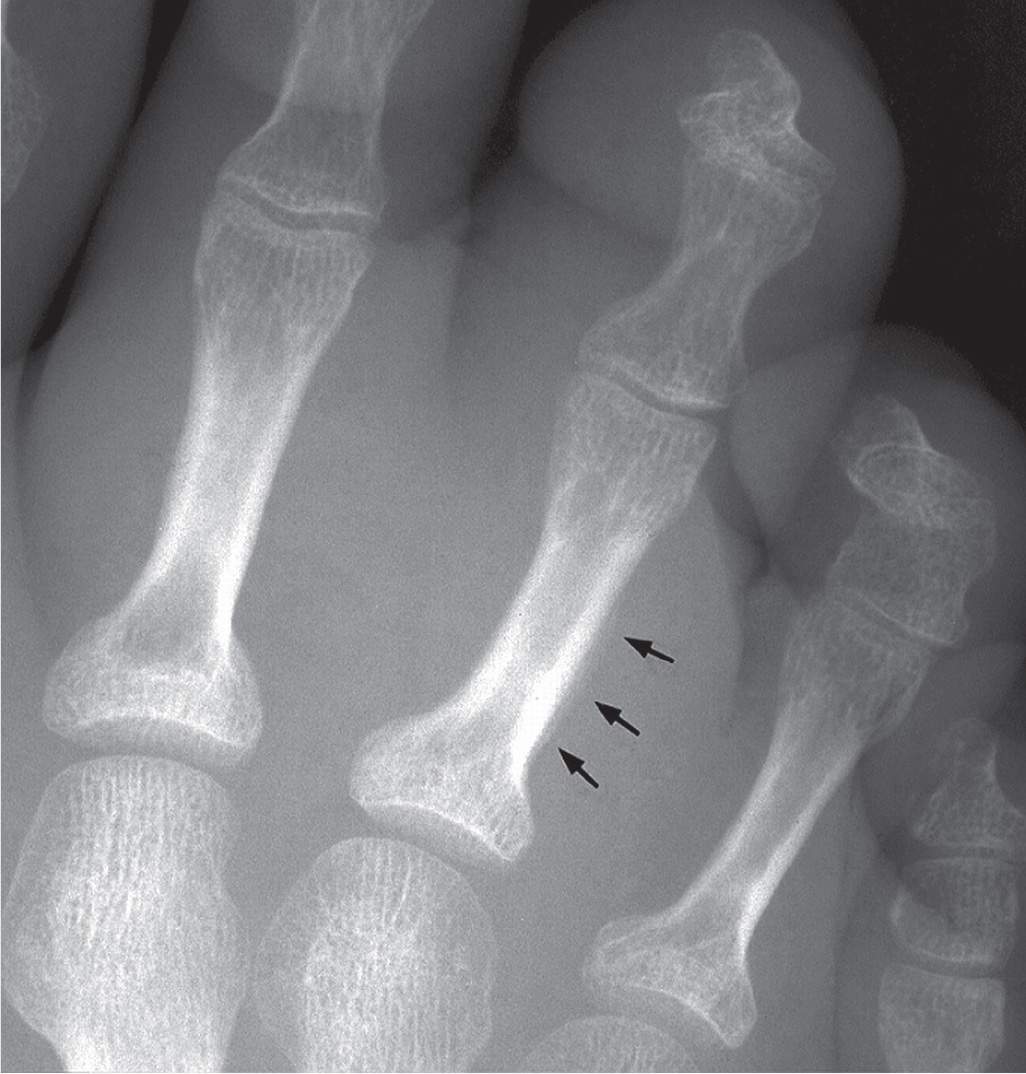
FIGURE 18-1. Infectious periostitis. A subtle, linear periosteal reaction (arrows) can be seen along the lateral aspect of the third toe proximal phalanx. Note the associated soft tissue increase in volume and density affecting the third and fourth toes.
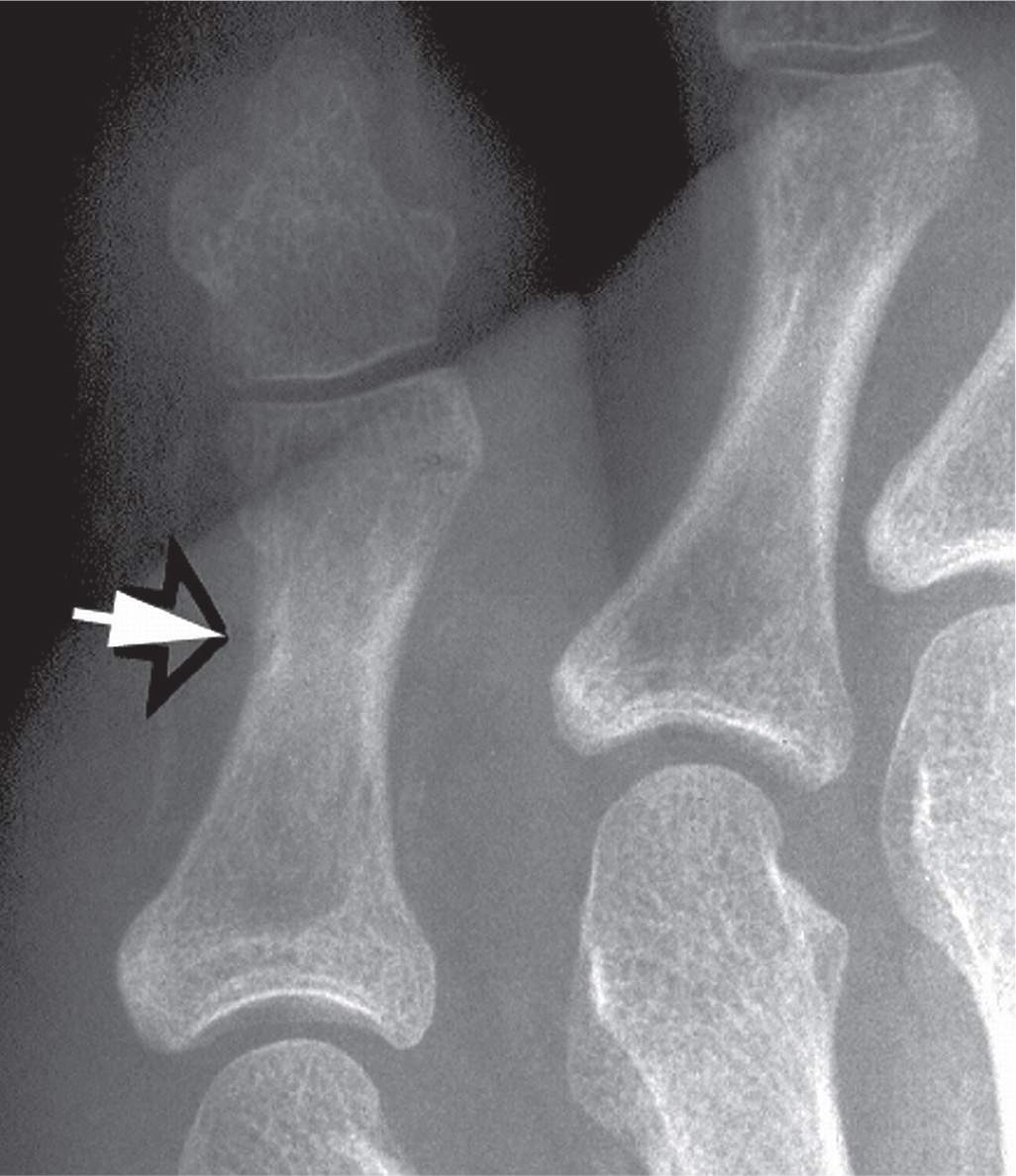
FIGURE 18-2. Infectious osteitis. Erosion (arrow) can be identified along the lateral aspect of the fifth toe proximal phalanx distal diametaphysis. No rarefaction or other lysis is seen radiographically that would suggest medullary involvement.
Clinical Presentation and Onset
The clinical stages of osteomyelitis may be acute, subacute, or chronic (Table 18-1).10 Acute osteomyelitis is clinically identified by an insidious onset of pain localized to bone, with soft tissue swelling and erythema of the involved area. A patient may develop fever, malaise, irritability, and marked increase in pain. During the initial stage of infection, it is difficult to ascertain the infectious process radiographically. Soft tissue swelling and obliteration of fascial planes may be the only radiographic signs (Figure 18-8A).11 The term rarefaction is used to describe localized loss of bone density. It is one of the earliest findings of osteomyelitis (Figure 18-8B).1,2,4,6 Despite the fact that periostitis is considered one of the “classic” findings of osteomyelitis,12 with the exception of hematogenous osteomyelitis, periostitis is often absent, especially in the toes. It may, however, be seen with less virulent infectious processes (Figure 18-1). The term osteolysis applies to a more destructive process or resorption of bone and usually follows localized rarefaction (Figure 18-8C).
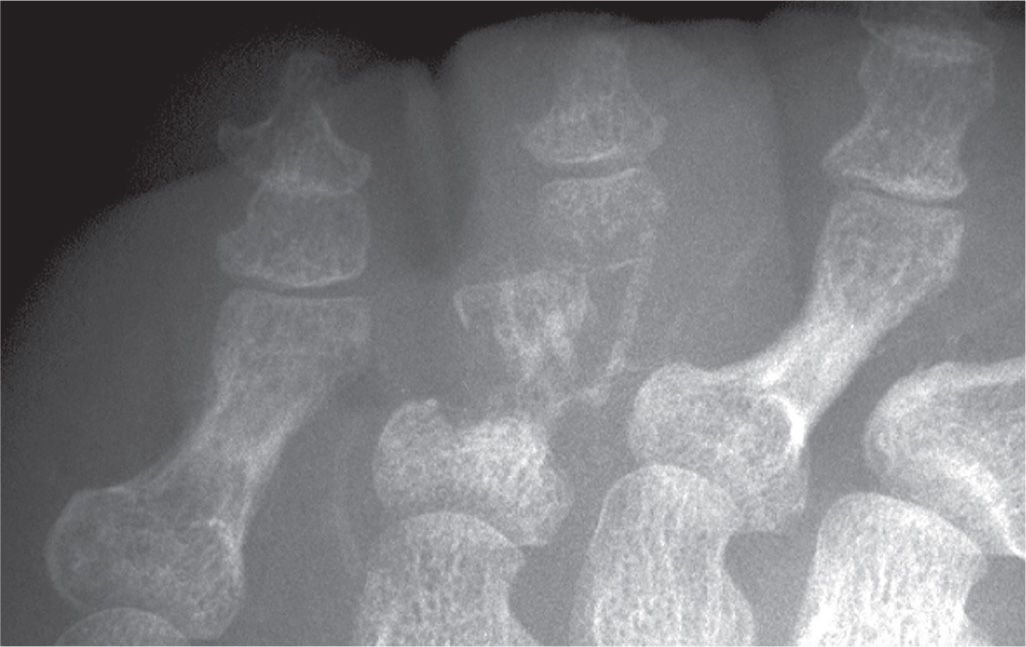
FIGURE 18-3. Osteomyelitis, fourth toe proximal phalanx. Infection involves the entire bone, resulting in loss of its form.
The term subacute osteomyelitis is used to describe a well-defined lytic lesion in bone that is caused by an infectious organism of low virulence.1,3 The walled-off abscess in bone known as Brodie abscess was first described by Sir Benjamin Brodie in 1832.13 A Brodie (bone) abscess can radiographically be differentiated from a benign bone cyst: the former appears as a geographic lytic area of bone with a dense sclerotic rim that fades peripherally (Figure 18-9).4 In contrast, the bone cyst will have a thin, well-defined sclerotic margin surrounding geographic destruction. The bone abscess may vary in size, from 1 to 4 cm in diameter, and is commonly found in metaphyseal bone.2
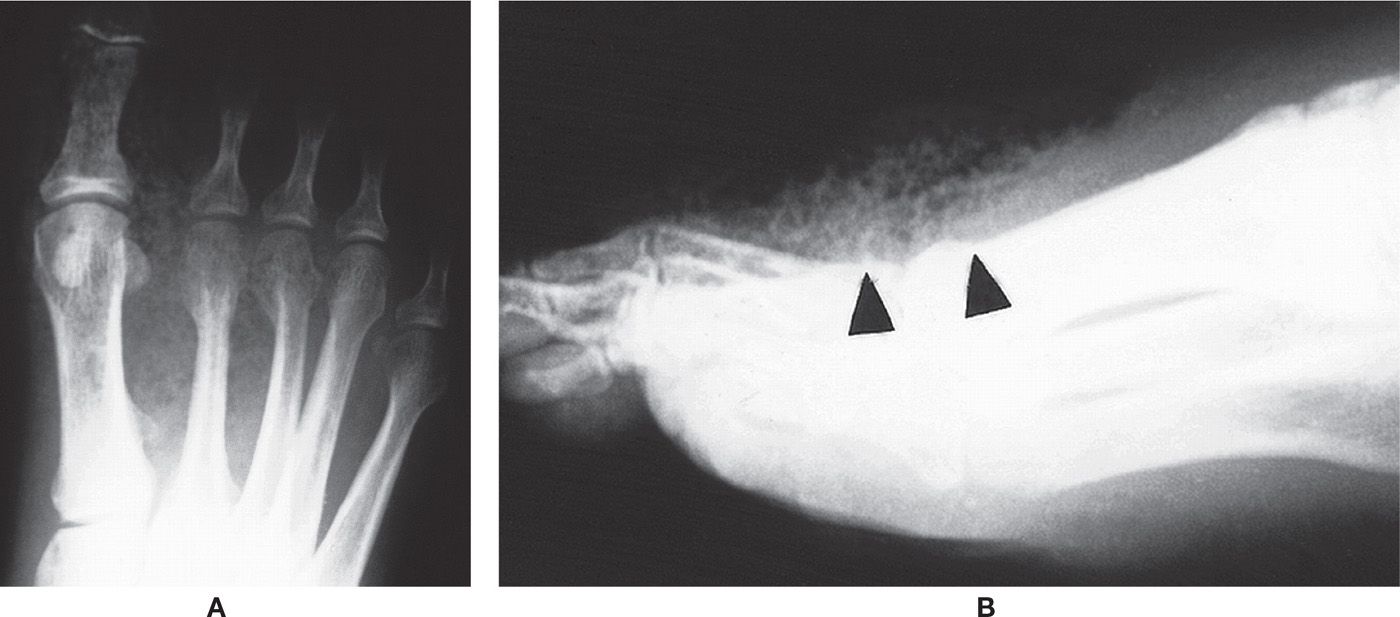
FIGURE 18-4. Soft tissue infection, gas gangrene. A: Dorsoplantar view. Air/gas is seen throughout the soft tissues of the forefoot. B: The lateral view shows loss of fascial planes dorsally as well as air/gas (arrowheads) in the soft tissues.
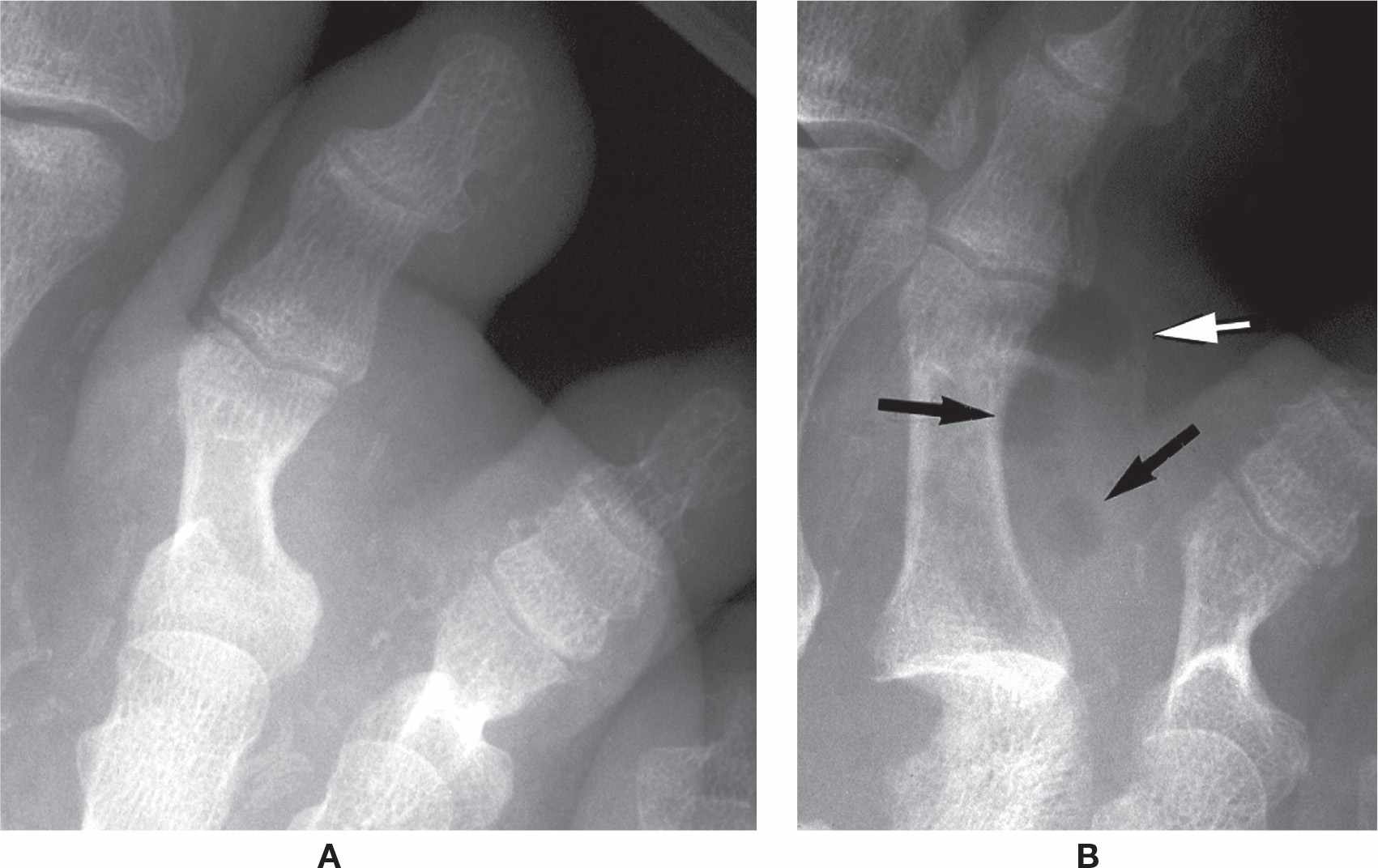
FIGURE 18-5. Soft tissue infection, second toe. A: Increased soft tissue volume and density affects the entire digit. B: Four weeks later, gas is evident (arrows).
Chronic osteomyelitis develops when an acute infection is inappropriately treated or therapy has been inadequate. It has been defined as an infection that has been present for at least 6 weeks.14 During the chronic process, viable new bone is laid down around dead bone, developing a periosteal envelope that can surround the entire shaft. This covering is called an involucrum (Figure 18-10).1,3,6 Therefore, involucrum surrounds dead bone and may give the remodeled bone an irregular or jagged outline. Long-standing chronic osteomyelitis results in significant bone deformity (Figure 18-11). Devitalized bone that is detached from the surrounding bone and necrotic due to the infection is known as a sequestrum.15 It may appear sclerotic relative to the viable surrounding area if the latter is osteopenic (Figure 18-11). Sequestrum is one of the most important findings in the assessment of chronic osteomyelitis. The presence of necrotic bone represents active infection during the chronic stage of the disease.1,3,6 The term cloaca refers to a defect in the cortex that allows pus and nonviable bone (sequestrum) to be expelled from the bone (Figure 18-12). Similarly, fistula or sinus tract is an opening that allows the sequestrum or pus to move through the soft tissues.
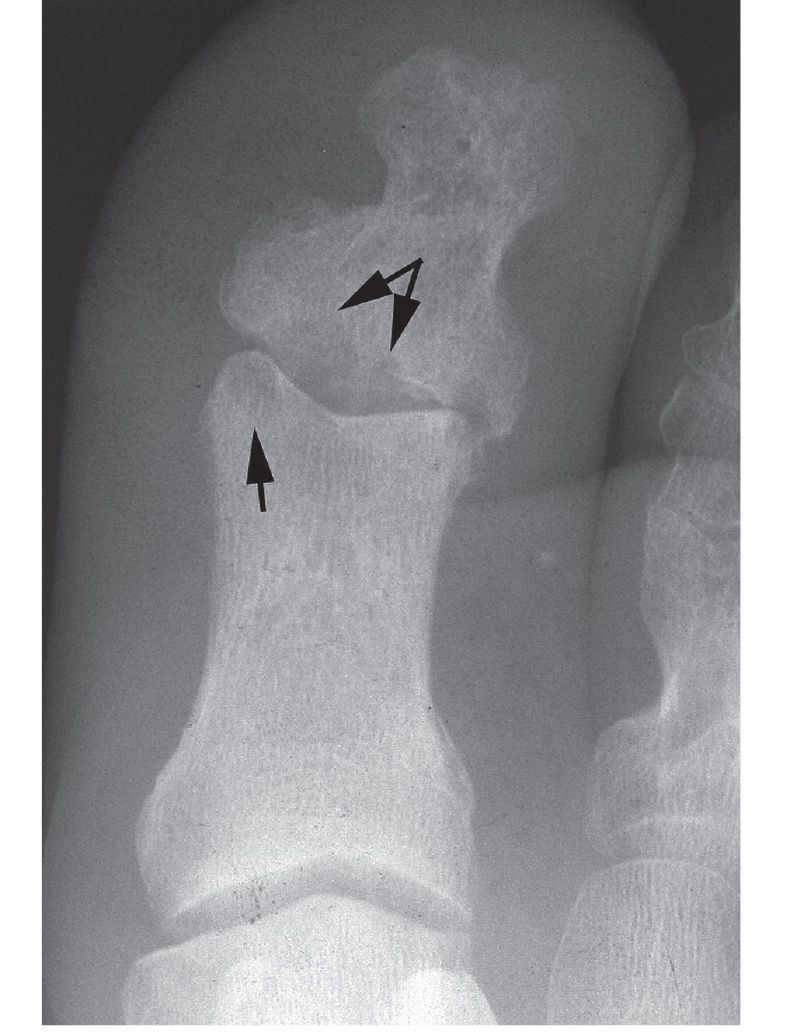
FIGURE 18-6. Septic arthritis, hallux interphalangeal joint. The primary finding is subchondral resorption (arrows). The joint space is slightly increased.
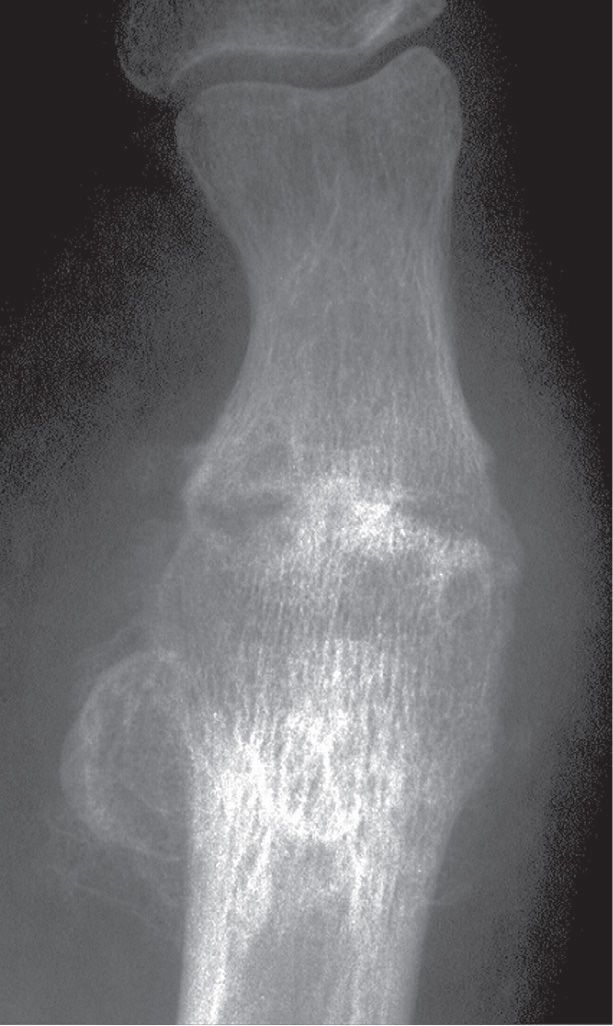
FIGURE 18-7. Ankylosis secondary to septic arthritis, first metatarsophalangeal joint. Significant joint space narrowing and subchondral resorption are seen along both sides of the first metatarsophalangeal joint. Bony union is occurring across the articulation.
| Classification of Osteomyelitis Based on Clinical Stages |
Clinical Signs | Radiographic Signs |
Acute Osteomyelitis | |
Insidious onset with pain, malaise, and fever | No radiographic findings initially; early findings may include periostitis, rarefaction, and increased soft tissue volume and density; eventually osteolysis |
Cellulitis | Obliteration of fascial planes |
Subacute Osteomyelitis | |
Low-grade pain with no systemic signs; occasional pain around the affected area | Well-defined lytic lesion in bone with dense sclerotic rim usually 1 to 4 cm in diameter (Brodie abscess) |
Chronic Osteomyelitis | |
Pain localized to the affected area, with soft tissue swelling and localized cellulitis; ulceration and draining; sinus may be present | Bone malformation with involucrum, cloaca, and sequestrum |
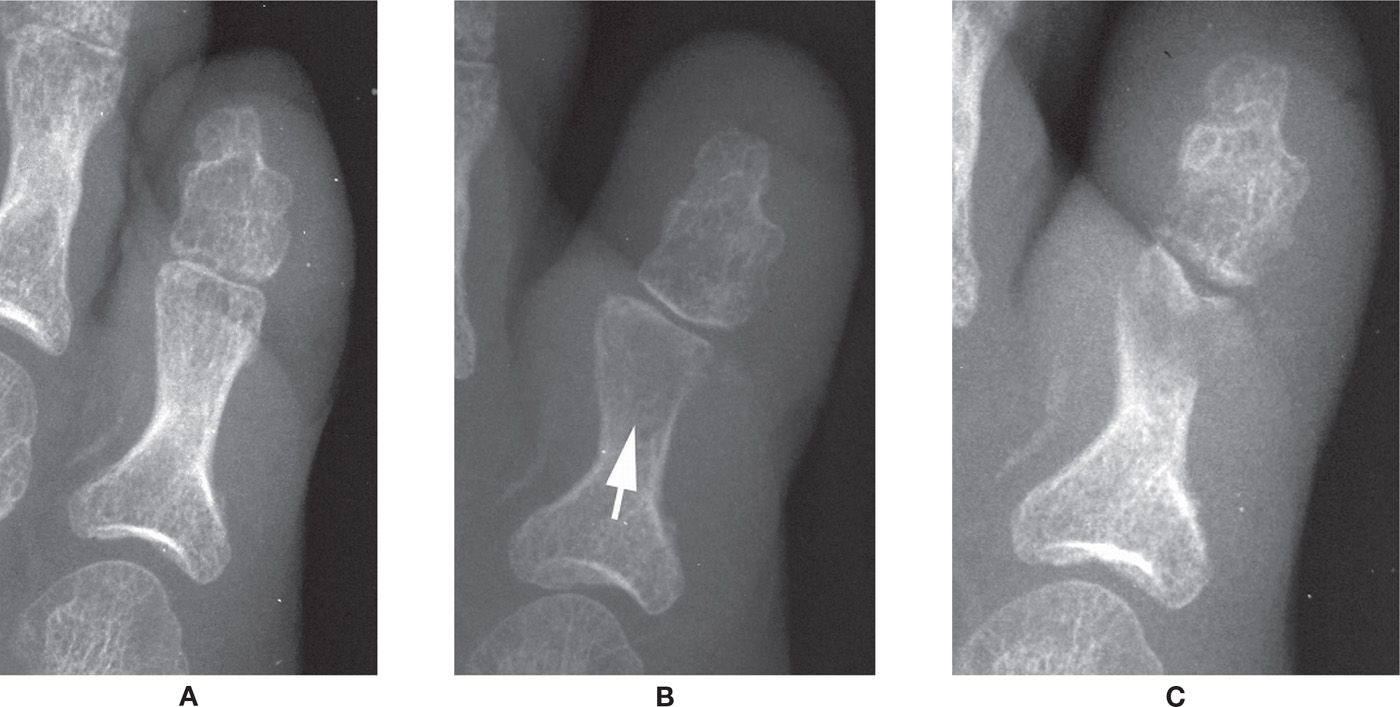
FIGURE 18-8. Progression of osteomyelitis, fifth toe proximal phalanx. A: Only finding is increased soft tissue volume and density. B: Significant rarefaction (arrow) is seen in the distal half of the proximal phalanx. Notice that the form of the phalanx is still intact. C: Osteolysis.
Sclerosing osteomyelitis of Garré is a chronic form of osteomyelitis caused by organisms of low virulence. The disease is nonsuppurative and usually affects a single bone. Radiographically the bone appears sclerotic and shows marked cortical thickening with very little evidence of a draining sinus or osteolysis. This entity must be differentiated from Ewing sarcoma, which is a more destructive lesion showing scalloping of the periosteum.1,3,6,16
Route of Transmission
Many authors have classified bone infection according to the route of contamination, which is important radiographically, because the findings may appear differently depending on the route.1,2,6,17 Different routes of contamination that cause osteomyelitis include osteomyelitis secondary to contiguous soft tissue; puncture wounds or implantation devices such as pins, wires, screws, or staples from surgery; postoperative wound infections; and hematogenous osteomyelitis.1 The Waldvogel classification is most familiar, and divides osteomyelitis into two modes of transmission: direct extension and hematogenous.17
Osteomyelitis secondary to contiguous soft tissue or direct extension to bone is more common than hematogenous osteomyelitis in the lower extremity and foot.2 Direct extension osteomyelitis is caused by an organism invading bone from the outside via a portal of entry.4,6 As a result, infection first contacts the periosteum, and resides between it and the cortex (infectious periostitis). If left untreated, the infectious process erodes the underlying cortex (infectious osteitis). After the periosteum is invaded and the cortex is destroyed, the infection will reach the marrow cavity and is now a full-blown osteomyelitis.
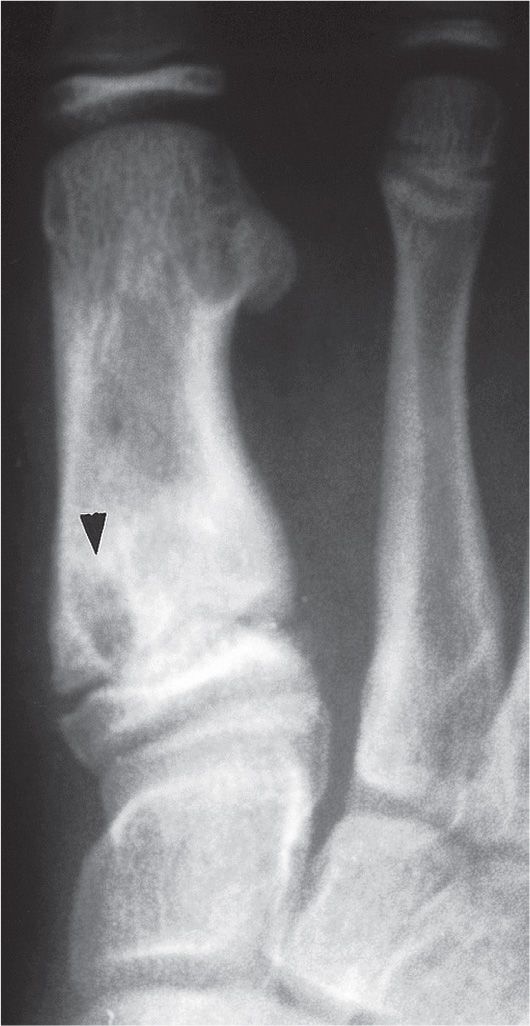
FIGURE 18-9. Brodie abscess, first metatarsal. This is an 8-year-old boy, 11 months after metatarsus adductus surgery with a painful first metatarsal. A geographic, lytic lesion lies at the medial aspect of the first metatarsal proximal metaphysis (arrowhead). This lesion represents a bone abscess. Note that the epiphysis is spared and the lesion has a sclerotic rim that fades peripherally away from the epiphyseal plate.
Initial radiographic findings associated with direct extension osteomyelitis, whether it be from contiguous soft tissue infection, puncture wound, or surgery, are increased soft tissue volume and density and obliteration of fascial planes. (The soft tissue radiographic abnormalities in hematogenous osteomyelitis occur later in its course.) As the infection invades the periosteum, it lifts the cortex and can stimulate formation of a periosteal reaction. If left untreated, it invades the cortex. As the infection traverses the Haversian and Volkmann canals, cortical erosion and rarefaction occur. Pus can infiltrate the medullary bone vascular supply as the bone marrow becomes affected; circulation to bone becomes sluggish, and osteolysis occurs.
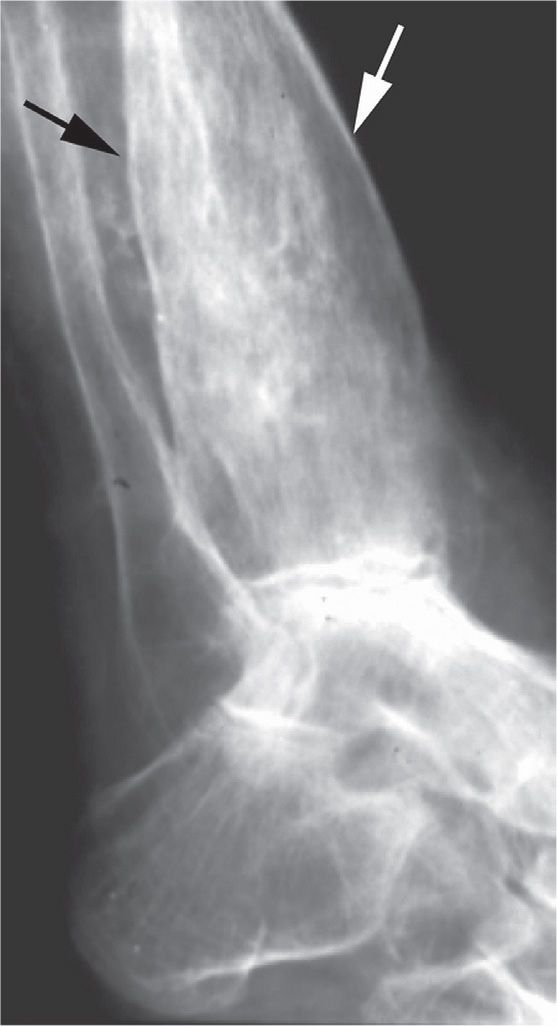
FIGURE 18-10. Chronic osteomyelitis, involucrum. The distal tibia is grossly deformed; the collar of remodeled bone surrounding the infected bone is known as an involucrum.
Osteomyelitis is difficult to differentiate from normal healing bone in the postoperative patient because a proliferative periosteal reaction may also occur in the latter (Figure 18-13). Early diagnosis is very important, however, to prevent an acute osteomyelitis or infectious periostitis from becoming a chronic problem. Other diagnostic modalities, such as white blood cell–labeled bone scintigraphy or magnetic resonance imaging (MRI), may help differentiate normal bone healing from infection. Increased osseous and/or cartilaginous destruction with radiolucency around an implant or prosthesis should alert the clinician to possible bone infection.1
A subtype of osteomyelitis from a contiguous focus of infection is whether or not there is vascular insufficiency.18,19 This is particularly important because of the fact that conditions such as diabetes fall in this category. The most common age group affected is that between 50 and 70 years of age. Osteomyelitis in this subtype usually develops from a localized infection or ulceration of the skin. Radiography of a patient with severe peripheral vascular disease and osteomyelitis may show minimal findings except for osteopenia caused by poor perfusion to bone in general. Osteomyelitis can be difficult to differentiate from Charcot neuropathic osteoarthropathy (Figure 18-14).20,21
Diabetic osteomyelitis is often associated with an ulcer or skin infection. Radiographs show increased soft tissue volume and density with or without gas or air in the tissue, depending on the type of organism present. Gas-producing organisms such as Clostridium and Bacteroides can lead to soft tissue necrosis and ultimately to gas gangrene. Obliteration of fascial planes is a radiographic sign associated with cellulitis. Periosteal new bone formation, rarefaction, subchondral resorption, and osteolysis can occur, as with exogenous osteomyelitis. Radiographic evaluation of osteomyelitis in the diabetic with underlying peripheral neuropathy and/or vascular disease can be confusing, because the radiographic features overlap (Figure 18-15). Furthermore, osteomyelitis and neuropathic osteoarthropathy can coexist (Figure 18-16). Nuclear medicine and MRI play an important role in differentiating osteomyelitis from Charcot neuropathic osteoarthropathy and can be used if the question arises. The radiographic picture of Charcot neuropathic osteoarthropathy is described in Chapter 19; the diabetic foot is the focus of Chapter 22.
Hematogenous osteomyelitis is an infection in which the bone is infected by blood-borne organisms that are deposited in medullary and metaphyseal regions of bone. It gives a somewhat different appearance from that of direct extension osteomyelitis. Metaphyseal bone is most often affected; it is more vascular and the sluggish, venous sinusoidal blood flow provides a good medium for bacterial growth, enhancing localization of the organisms in the metaphysis and marrow.22,23 This is especially true in children, in whom nutrient arteries are relatively large, and the branches to the marrow, cortex, and metaphysis are small end arteries or capillaries that are relatively stagnant.22 Intraosseous pressure rapidly increases with infection, leading to marked necrosis and demineralization. If the infection persists, exudate continues to expand and spread through the Volkmann and Haversian systems of bone. Blood flow is increasingly disrupted, leading to advanced necrosis, resorption, and possibly sclerosis. As the infection advances, it crosses the cortex, with ensuing periosteal proliferation. Subperiosteal abscess formation and subsequent irritation may lead to new bone formation or involucrum, if chronic.4,7,22 Subperiosteal proliferation may be noted as early as 5 to 7 days in children, whereas in adults it may appear in 10 to 14 days; it is usually a more common finding in children because the periosteum is loosely attached. In addition, large areas of dead bone (sequestra) may be surrounded by granulation tissue and walled-off from adjacent viable bone.
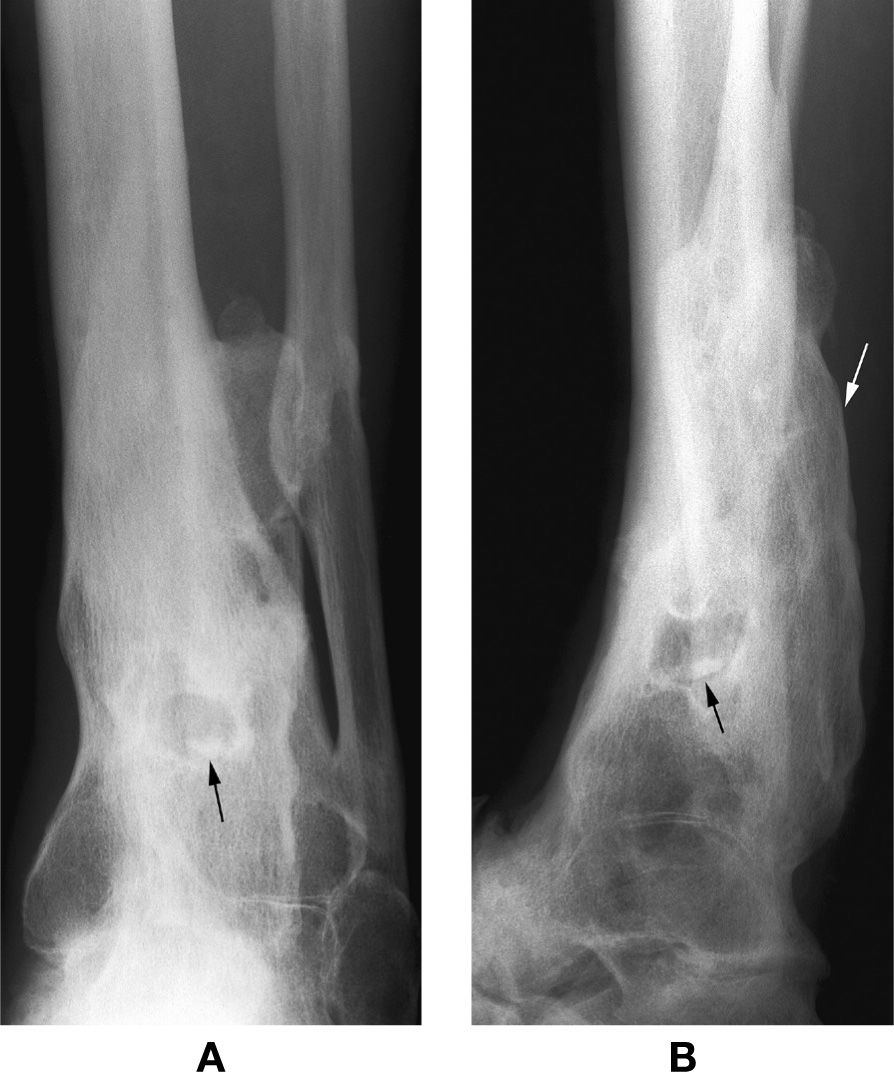
FIGURE 18-11. Chronic osteomyelitis, distal leg. A: Anteroposterior view. B: Lateral view. After many years, the periosteal remodeling (involucrum, white arrow) yields severe bone deformity. The black arrow identifies a probable sequestrum, oval-shaped and sclerotic. CT may be necessary to document its separation within the infected bone.
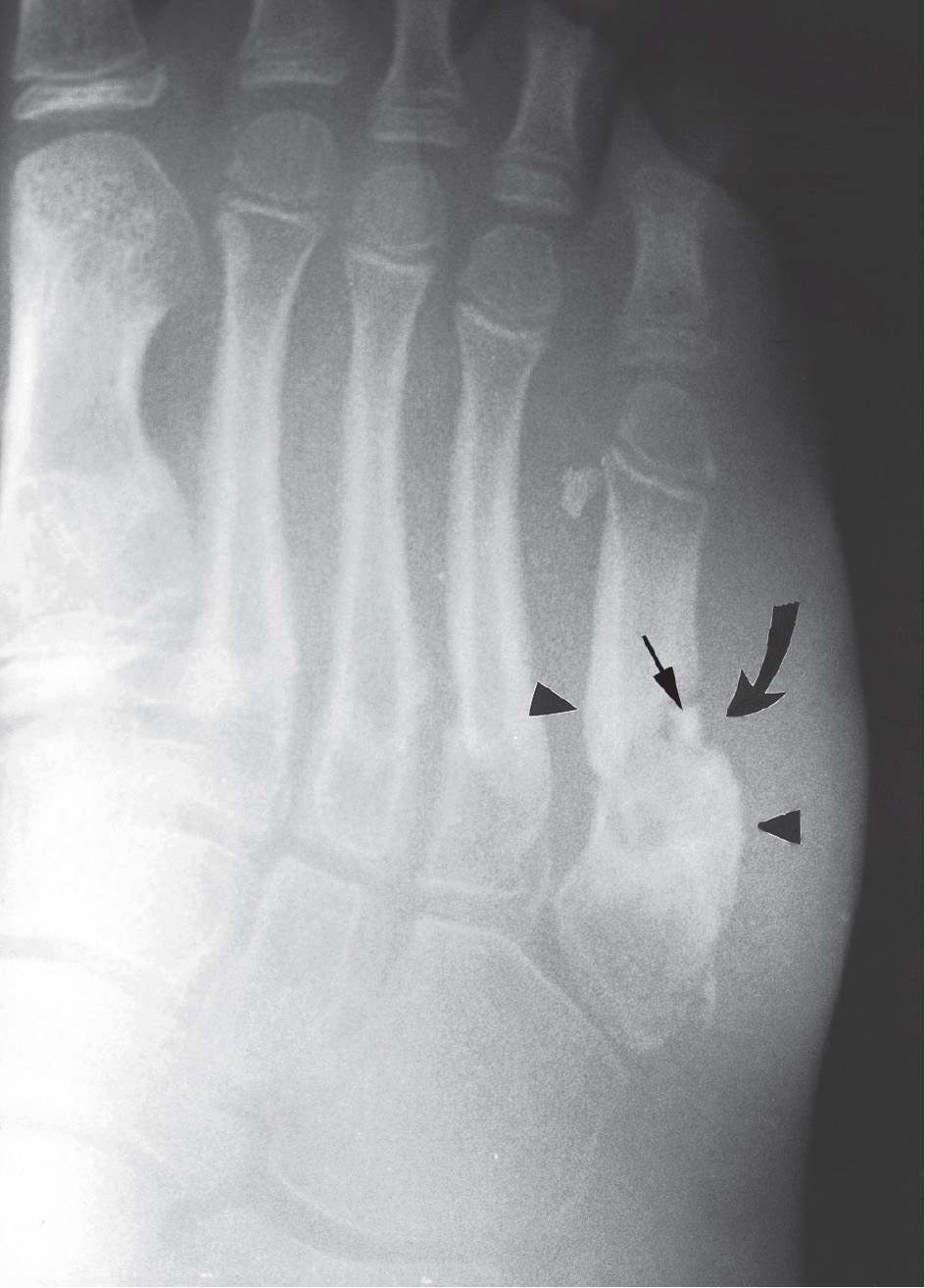
FIGURE 18-12. Chronic, active osteomyelitis. Note the well-defined lytic lesion within the base of the fifth metatarsal. Also note the extensive periosteal thickening and remodeling (arrowheads), representing an involucrum. The involucrum surrounds or walls off the sequestra within the cyst like lesion. A small sequestrum (straight arrow) is exiting through a cloaca (curved arrow).
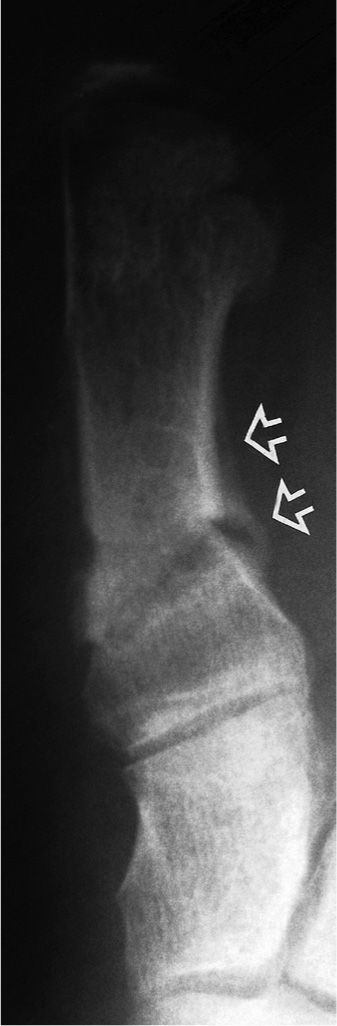
FIGURE 18-13. Normal postoperative periostitis. A periosteal reaction is seen along the lateral aspect (open arrows) of the first metatarsal proximal metadiaphysis. This radiograph was performed 13 weeks after a base wedge osteotomy with screw fixation. The screw was removed because it loosened. The periosteal reaction could represent infection, but this example proved to be an uninfected periostitis secondary to normal healing.
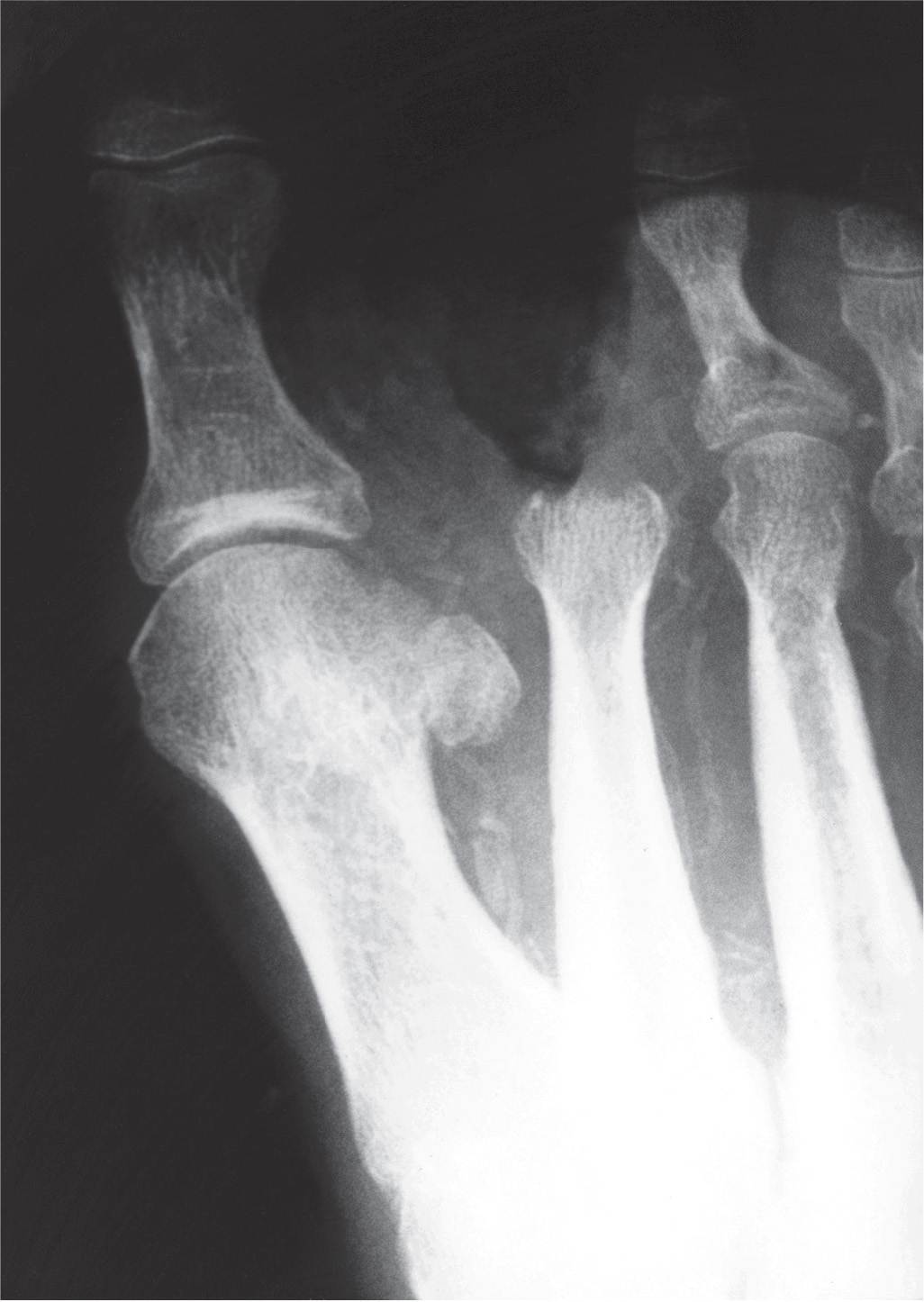
FIGURE 18-14. Osteomyelitis versus neuropathic osteopathy, second metatarsal head. This diabetic patient previously had amputation of the second toe because of severe vascular compromise. Subchondral resorption is now evident along the distal aspect of the metatarsal head. In most cases, diabetic osteopathy cannot be easily differentiated from osteomyelitis radiographically.
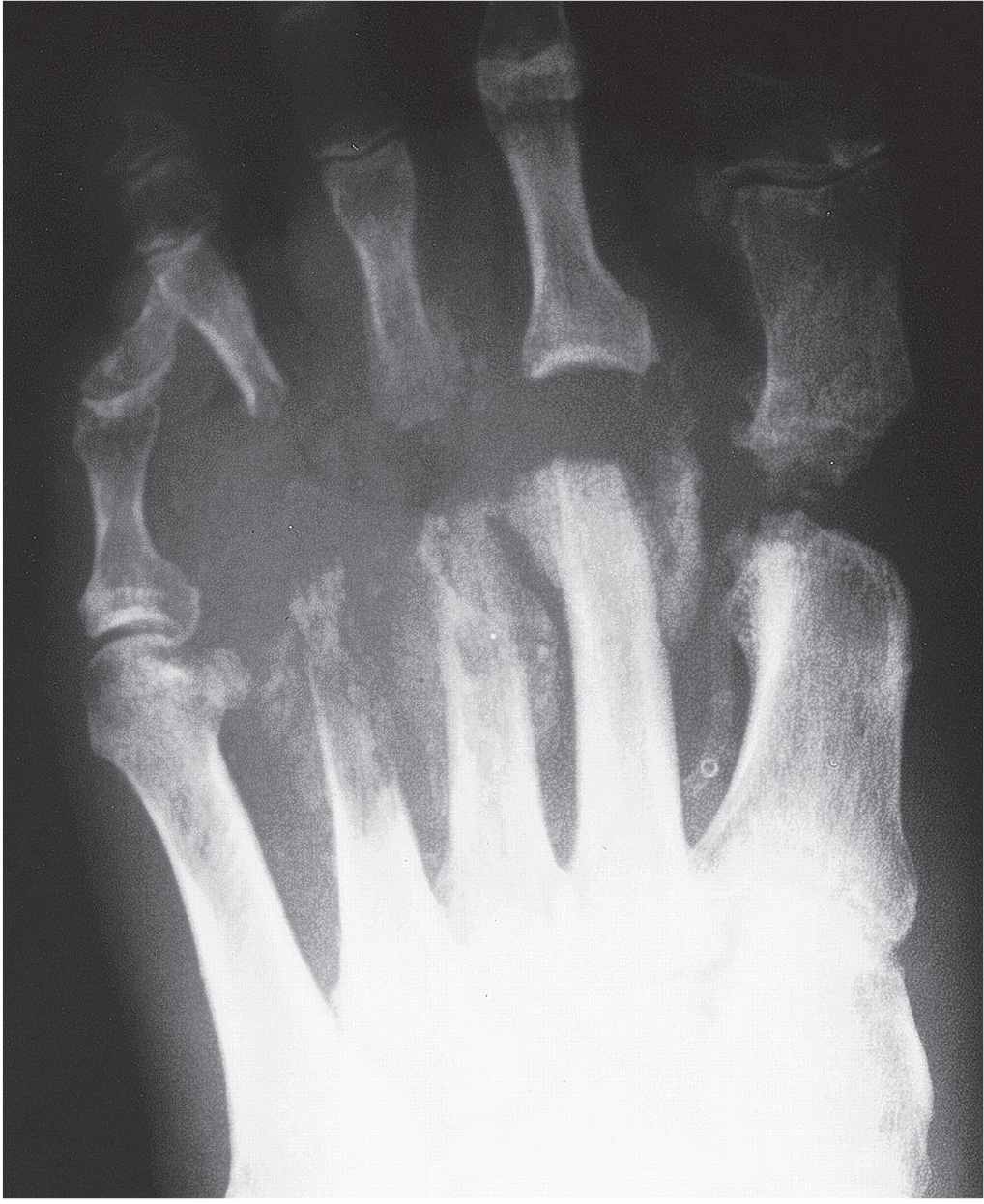
FIGURE 18-15. A diabetic patient with severe peripheral vascular disease. In the proper clinical setting, soft tissue edema, rarefaction, osteolysis, erosion, and periosteal new bone production all suggest osteomyelitis.
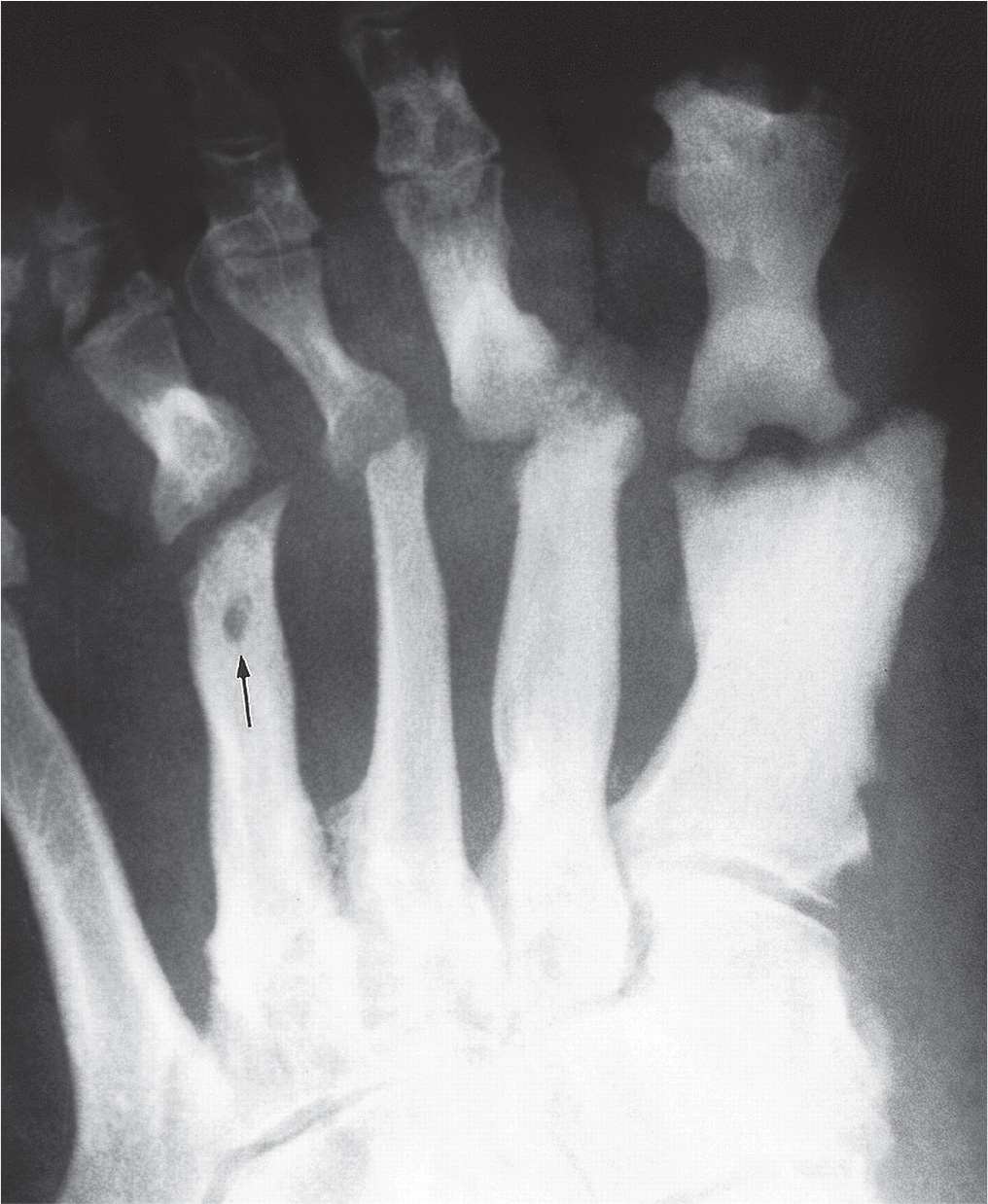
FIGURE 18-16. Mixed long-standing osteomyelitis and neuropathic osteopathy in a diabetic patient. Exuberant remodeling involves the first four metatarsals and adjacent proximal phalanges. The geographic, lytic lesion surrounded by diffuse sclerosis in the fourth metatarsal distal diaphysis is a bone (Brodie) abscess (arrow).
Patient Age
Metaphyseal vascular anatomy is age dependent and subsequently influences the radiographic presentation of hematogenous osteomyelitis (Figure 18-17). Trueta22 has described three distinct patterns of osteomyelitis based on metaphyseal blood supply in infants, children, and adults.
An infantile pattern is described in children less than 1 year of age. Metaphyseal vessels penetrate the epiphyseal growth plate in the infant to supply the epiphysis. Subsequently, metaphyseal infection may cross the growth plate, invade contiguous joint spaces, and result in sepsis. Neonatal osteomyelitis rapidly transgresses the growth plate and leads to septic arthritis, which can destroy the joint as well as bone.5,22,17 Involvement of the growth plate may lead to a decrease in the length of the affected limb. When the epiphyseal plate is spared, hyperemia may accelerate growth rate, with early maturation and closure of the growth plate.6
The juvenile pattern affects children from 1 year of age to closure of the physis. No vascular penetration of the growth plate occurs during this period; acting as a “barrier,” it confines the infection to the metaphyseal region (Figure 18-18). Infection may spread laterally, however, perforating the cortex and elevating the loosely adhered periosteum.
The adult pattern is evident after growth plate closure. Infection can penetrate into subchondral bone and joint sepsis is more likely. Because the periosteum adheres more firmly to underlying bone in the adult, less periosteal reaction is seen than in the child.
Anatomic Type
Cierny et al.24 proposed a classification system that considers the bones anatomic nature, the host quality, as well as treatment and prognostic factors. This system, however, may not be as useful for the toes or other small bones.25 There are four anatomic stage types: medullary, superficial, localized, and diffuse osteomyelitis.
There is soft tissue compromise in both the medullary and superficial locations. The nidus of medullary osteomyelitis is endosteal; however, superficial osteomyelitis has a contiguous focus on the bone’s surface. Localized osteomyelitis involves the full thickness of bone and includes the periosteum, cortex, and medullary canal; its etiology is typically direct extension. Cierny et al.24 state that localized osteomyelitis is often a combination of medullary and superficial findings. Diffuse osteomyelitis is defined as a “permeative, circumferential, or through and through disease of hard and soft tissue.”24
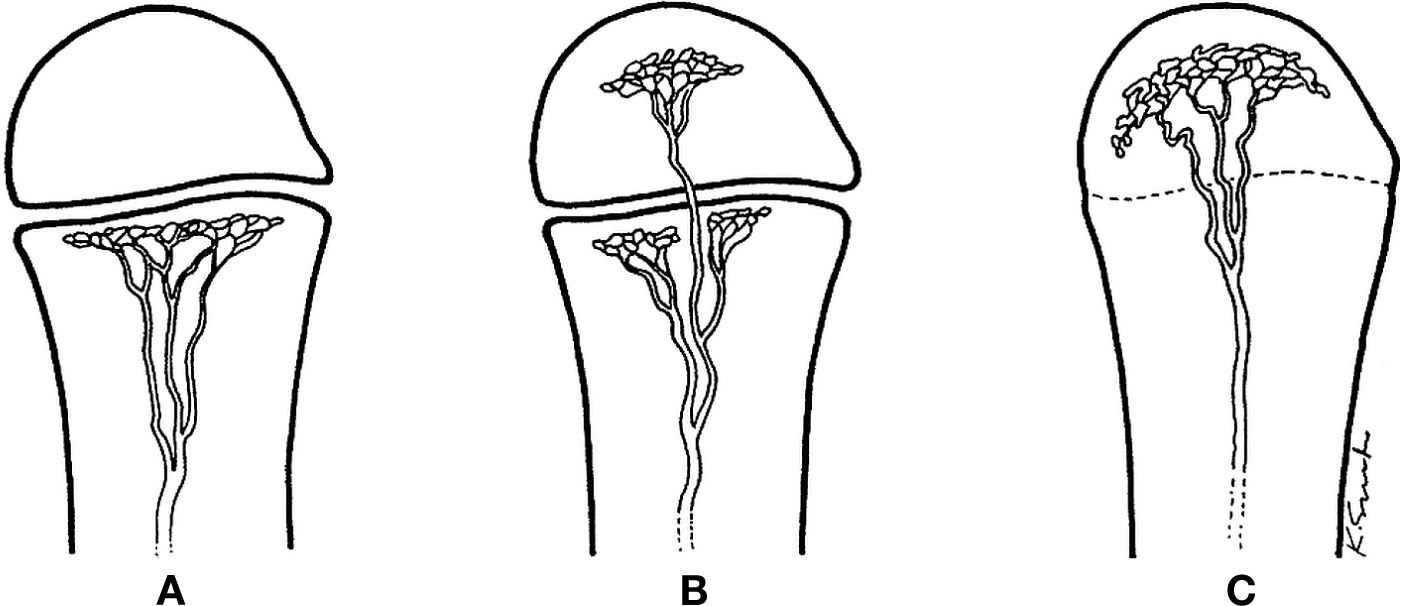
FIGURE 18-17. Normal vascular patterns of a tubular bone in the child, infant, and the adult. A: In the child, the capillaries of the metaphysis turn sharply, without violating the open growth plate. B: In the infant, some metaphyseal vessels may penetrate the open growth plate, ramifying in the epiphysis. C: In the adult, with closure of the growth plate, a vascular connection between metaphysis and epiphysis can be recognized. (Courtesy of Resnick D. Diagnosis of Bone and Joint Disorders. Philadelphia, PA: Saunders; 1981:2048 [Figure 60-2].)
The Infecting Organisms
Staphylococcus aureus is the most common infectious agent in all age groups.8,26 Organisms most commonly causing acute hematogenous osteomyelitis usually stem from a single organism such as Staphylococcus or Streptococcus. In young adults Staphylococcus aureus is considered the primary infecting organism, whereas in the elderly gram-negative rods are most commonly isolated. Pseudomonas aeruginosa and methicillin-resistant Staphylococcus are frequently found in the intravenous (IV) drug abuser.
Osteomyelitis secondary to contiguous soft tissue or by direct extension depends on the mechanism of transmission. For example, a puncture wound is most commonly affected by Staphylococcus or Pseudomonas, whereas an animal bite from a dog or a cat can cause an infection from Pasteurella multocida. Pseudomonas is also seen in nosocomial infections.26
Diabetics tend to contract polymicrobial wound infections leading to osteomyelitis. Aerobes as well as anaerobes must be considered. The primary infecting organism is most commonly isolated by bone biopsy. A culture from the sinus tract or ulcer is usually affected with mixed flora and can be unreliable for proper diagnosis and treatment of the bone infection.27
Chronic osteomyelitis can be caused by many organisms, including bacteria, fungi, mycobacteria, and the spirochete Treponema pallidum (which causes syphilis). Osteomyelitis secondary to Mycobacterium tuberculosis can be identified radiographically as well as clinically because of the chronicity of the disease. Clinically the patient has pain, stiffness, mild-to-moderate swelling, and erythema of the affected part. The soft tissues are affected by a granulomatous reaction leading to severe soft tissue destruction. The soft tissue destruction is characterized by mononuclear cell infiltrates, giant cell inflammatory infiltrates, fibroblast proliferation, mild edema, and small vessel congestion.28,29
Radiographic findings of osteomyelitis secondary to M. tuberculosis include soft tissue changes early on followed by bone destruction. Bone destruction is usually evident, because of the chronic nature of the disease. Bone changes include subchondral osteopenia followed by irregular areas of destruction with minimal marginal sclerosis. Periosteal reaction is characteristically minimal. Joint changes may occur, and spaces between joints may narrow. Damage to epiphyseal plates can cause growth abnormalities in children. Sequestrum is much less common with M. tuberculosis than in osteomyelitis from a bacterial origin.30,31
Fungal osteomyelitis may mimic bacterial or tuberculosis infection of bone both clinically and radiographically. Clinicians usually consider the possibility of a fungal infection of bone when a patient has not responded well to antibiotic therapy for a bacterial infection or bacteria have been isolated from the bone culture.29
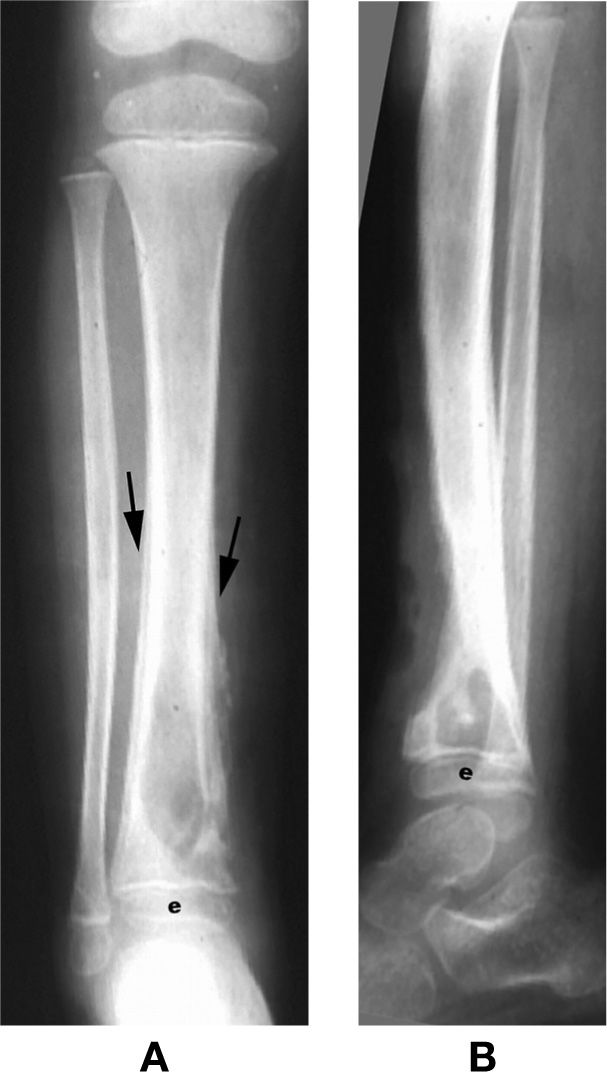
FIGURE 18-18. Hematogenous osteomyelitis, juvenile pattern. A: Anteroposterior view. B: Lateral view. There is significant rarefaction involving the metaphysis and adjacent diaphysis; however, the epiphysis (e) is unaffected. Arrows identify exuberant periosteal reaction.
Syphilitic osteomyelitis can be either congenital or acquired with tertiary syphilis. In the fetus, the newborn, or young infant, bone destruction can develop. A syphilitic osteochondritis can occur, causing changes in endochondral ossification. This leads to broad, horizontal radiolucent bands on a radiograph. Metaphyseal abnormalities can appear, leading to epiphyseal separation. Diaphyseal osteomyelitis can appear in the untreated newborn or infant. Osteolytic lesions with bony eburnation and underlying periostitis are found in the diaphyseal bone. Periostitis can also be seen in congenital syphilis. It is diffuse, symmetric, and widespread.1
Acquired syphilis can cause bone destruction as well. The bone changes usually occur in the tertiary stage of the disease. Irregular cortical thickening may be present. The tibia and femur are the most commonly affected bones in the lower extremity. The irregular cortical thickening of the anterior tibia has been described as a “saber shin” appearance.16 Periosteal reactions may be irregular or lacelike and show spicules radiating perpendicular to the shaft of the bone, mimicking osteogenic sarcoma.1,16
SPECIAL IMAGING MODALITIES
This section provides an overview of special imaging studies and their application to skeletal infection in the foot. Adjunctive studies are discussed in Section 6, Special Imaging Procedures, and the reader is encouraged to refer to those chapters for further information.
Radiography remains the initial study of choice for imaging infection. If there is obvious evidence of osteomyelitis radiographically in the patient who has not had surgery recently performed at the site and there is no other superimposed pathology (fracture, arthritis, tumor, etc.), there is no need to perform additional adjunctive studies unless considering surgical intervention. However, when the diagnosis is not obvious or is compounded by superimposed pathology, several adjunctive studies are available (Table 18-2).
Special imaging modalities are extremely helpful in differentiating osteomyelitis from soft tissue infection, underlying neuropathic disease, or postoperative bone healing, which all may mimic osteomyelitis radiographically with increased soft tissue volume and density, rarefaction, periosteal reaction, erosion, subchondral resorption, or osteolysis. MRI is probably the most widely ordered imaging study after radiography, but radionucleotide techniques are still used for the early detection of osteomyelitis and in patients where MRI is not available or contraindicated. In addition to CT, ultrasound and positron emission tomography (PET) scan are being used to aid in the diagnosis of osteomyelitis. Often a combination of imaging studies is used to confirm the presence of infection.
Nuclear Medicine
Radionucleotides that can be used for detecting osteomyelitis include technetium-99 ethylene diphosphonate (99mTc-MDP), gallium-67 citrate, and indium-111 (In-111). Localization of Tc-99 is related to both osteoblastic activity and skeletal vascularity. A technetium bone scan can often be positive 24 hours after the onset of symptoms and 10 to 14 days before any radiographically visible changes have occurred.32 A positive scan shows a well-defined, localized, increased uptake of Tc-99m in an area of inflammation or infection, also referred to as a “hot spot”; there will be increased uptake in all three phases, the angiogram, blood pool phase, and the actual bone scan (Figure 18-19). A positive bone scan does not prove that osteomyelitis is present, because Tc-99m uptake is relative to osteoblastic activity and therefore anything that causes increased osteoblastic activity produces a positive scan. Occasionally, 1 to 2 days after development of symptoms due to infection a technetium scan may be normal or show an area of decreased uptake or a “cold spot” because the medullary microcirculation is compressed by intraosseous pus.11 A cold spot shows up as a lack of radionucleotide accumulation in the affected bone area. Triphasic bone scintigraphy cannot differentiate osteomyelitis from other causes of active bone remodeling such as recent fracture, neoplasm, loose prosthesis, septic arthritis, or diabetic osteoarthropathy.33 For this reason, other nuclear scans have been used with technetium to help in making a diagnosis in those instances (Figure 18-20A).
Primary Use of Imaging Studies for Osteomyelitis |
Imaging Method | Use | Sensitivity/Specificity (%)a |
Radiography | Initial study of choice | 43–75/75–83 |
Ultrasound | Soft tissue abscess; periosteal elevation; guidance for biopsy, incision and drainage | |
MRI | Early detection when radiograph unremarkable; after diagnosis, useful for determining extent of involvement; preoperative planning; diabetic foot | 82–100/75–96 |
CT | Detecting/documenting sequestra in chronic osteomyelitis | 67/50 (chronic osteomyelitis)b |
Bone scintigraphy (three-phase bone scan) | Early detection; identifying multifocal involvement | 85/≈25 |
Bone scan + gallium | Increases specificity of bone scan for early detection | ≈60/≈80 |
White blood cell scan (indium, Tc-HMPAO) | For complicated osteomyelitis when there is superimposed pathology | |
FDG-PET | Confirm or exclude chronic osteomyelitis | 98/91 (chronic osteomyelitis) |
aFrom Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg. 2009;23:80.
bFrom Termaat MF, Raijmakers PG, Scholtein HJ, et al. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:2464.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








