16 WOUND HEALING AND SOFT TISSUE INJURIES
The skin is the enveloping external organ of the human body and it is the first line of defense in any injury. Thus the skin is nearly always injured when trauma occurs. The extent of traumatic wounding varies from minor abrasions to large gaping wounds. Procedures and surgical interventions to treat injury also may create punctures, incisions or large openings in the skin. Injury to the skin and soft tissues predisposes the individual to secondary complications such as (1) localized and systemic infection, (2) hypoproteinemia, (3) hypothermia, and (4) sequelae related to tissue necrosis. Minimizing the risk of secondary complications and maximizing wound healing are optimal goals of all trauma patients with soft tissue injury.
ANATOMY OF SKIN
The skin has a surface area of 1.5 to 2 m2 and accounts for one sixth of total body weight, making it the largest organ of the body. It is one of the fastest growing tissues of the body, evidenced by complete replacement every 4 to 6 weeks. Healthy skin is critical to survival through its prevention of dehydration, its function as a barrier to external insults such as chemicals and microorganisms, and its role in the provision of thermal regulation. Regulation of body temperature is aided by the skin through covering and insulating the body to prevent heat loss, sweating to cool the body, and cutaneous vasodilation and constriction to release or retain body heat. The skin receives about one third of the circulating blood volume and plays an important role in maintaining homeostasis. The skin also provides sensory feedback about pressure, thermoperception, touch, and pain. Finally, body image, personal self-perception, and cultural values are affected by an individual’s physical appearance. Traumatic injury, specifically traumatic wounds and scarring, can influence a person’s self-perception and body image.1
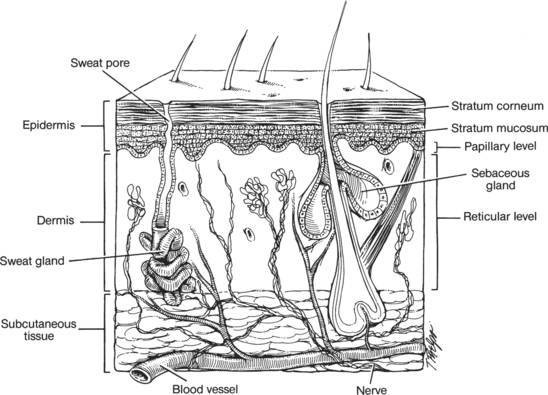
The integumentary system is composed of the skin and its appendages: hair, nails, and sweat and sebaceous glands. The skin is divided anatomically into two major layers: the epidermis and the dermis. The epidermis is the external protective layer, and the dermis, composed largely of collagen and elastic fibers, provides strength, elasticity, and protection against mechanical shearing forces. The epidermis is the outermost layer of the skin. It is further divided into the stratum corneum (cornified layer), stratum lucidum (clear layer), stratum granulosum (granular layer), stratum spinosum (prickle cell layer), and stratum basale (basal layer). The stratum corneum, which makes up the most superficial skin layer, is composed of nonviable keratinized cells, desiccated cells that are shed continually. The stratum corneum is also referred to as the horny layer. The stratum lucidum, located below the stratum corneum, is the transitional layer. Cells in this layer release lipid granules into extracellular spaces before movement to the stratum corneum layer. This lipid-rich coating protects the epidermis against aqueous solutions. The stratum granulosum lies below the stratum lucidum and is known as the granular layer. The next layer is the stratum spinosum, in which cells have spinelike structures that create bridges between them.2 Stratum basale, also called the stratum germinativum or basal layer, is the mitotically active layer. Keratinocytes divide and begin the process of differentiation in this layer. This single layer of cells runs along the dermis, creating epidermal rete ridges. These cells engage in mitotic division in response to multiple stimuli, such as growth factors and chemoattractants (e.g., cytokines). The columnar basal cells undergo continual mitosis and are the source of new cells that eventually reach the stratum corneum. The epidermis is avascular, receiving nutrients from the blood vessels in the dermis and subcutaneous tissues.3,4
Migrating cells—melanocytes, Merkel cells, and Langerhans’ cells—are distributed uniformly throughout the basal and suprabasal layers of the epidermis.1–4 Melanocytes are responsible for producing melanin and giving rise to skin color. Carotene, oxyhemoglobin, and circulating substances in the plasma (e.g., bilirubin) are also present in the epidermis and will influence skin color. Merkel cells make up a small part of the basal layer and are believed to participate in the sensation of touch. Langerhans’ cells are macrophages that function primarily in delayed hypersensitivity reactions. They also produce interleukin-1, which aids T-cell activation2,4,5 and thus serve an important immune function. The basement membrane zone lies below the basal keratinocytes and is a very thin layer separating the epidermis from the dermis. The basement membrane provides mechanical support for the epidermis and allows for the transport of material between the layers.
The dermis lies between the epidermis and the subcutaneous tissue. The dermis is a connective tissue composed of fibrous proteins (collagen and elastin). The junction between epidermis and dermis is undulated with upwardprojecting dermal papillae and downward-projecting epidermal rete ridges. The dermis nourishes the epidermis through its rich supply of vascular and lymphatic structures. There is a superficial layer, the papillary dermis, composed of interlacing fine collagen fibers, blood vessels, nerve endings, and thermoreceptors, and a deeper reticular layer of thicker bundles of collagen that provide the skin with structural support (Figure 16-1). Fibroblasts, located in the dermis, are the major differentiating cell type that becomes active during the wound-healing process. Fibroblasts are responsible for the secretion of collagen and elastin. Sensory receptors within the papillary dermis respond to pain, cold, heat, touch, and pressure. Dermal appendages, hair follicles, and sweat glands reside within the reticular dermis and extend upward through the epidermis to serve as an important source of epidermal regeneration during wound healing.
The subcutaneous tissue lies between the lower border of the dermis and the deeper fascia and muscle tissues. Although not generally considered a true part of the skin, it is closely associated with the dermis and is an important tissue to consider in terms of wound healing. The subcutaneous tissue functions to absorb shock, insulate, store nutrients, and shape the body contour. It is composed of many cells, including adipocytes, histiocytes, plasma cells, lymphocytes, and mast cells. Fat lobules of the subcutaneous tissue are surrounded by strands of collagen that contain nerves. Vascular and lymphatic networks travel from the fascia through the subcutaneous tissue and supply the dermis. There are few vascular connections between fat lobules and neighboring structures, which leaves the subcutaneous tissue vulnerable to decreased perfusion.2–5 Altered vascular supply, or hypoperfusion of the subcutaneous tissue, has implications for wound healing. Complications of impaired healing such as infection often have their origin in subcutaneous tissue.
NERVES
Nerve injuries can be classified according to the Sunderland system (Table 16-1), which prognosticates the time and degree of functional recovery.6 The extent of nerve damage is often unclear at the initial evaluation and can be surmised only by neurometric tests and serial examinations to evaluate functional progress.
PHYSIOLOGY OF WOUND HEALING
When the skin or internal organs are disrupted by trauma or surgery, a series of interdependent physiologic events occur that result in tissue repair (Figure 16-2).7 The wound healing process is frequently described as three overlapping phases of healing—inflammation, proliferation, and remodeling. The tissue response has several major components: (1) hemostasis, (2) inflammation, (3) epithelialization, (4) angiogenesis, (5) fibroblast proliferation, (6) matrix deposition, (7) contraction, and (8) remodeling.2–5 Through these responses, the process of wound healing is initiated, directed, and finally completed.
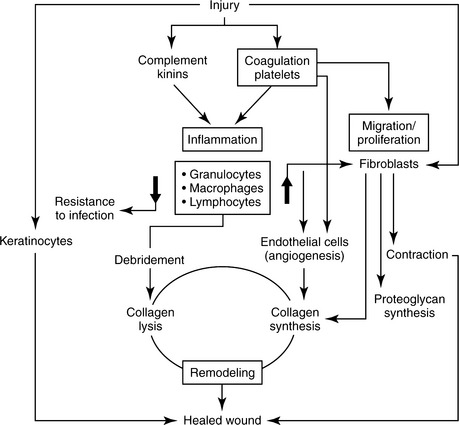
FIGURE 16-2 Schematic representation of wound healing.
(From Hunt TK, Hopf HH, Hussain Z: Physiology of wound healing, Adv Skin Wound Care 13:6-11, 2000.)
Tissue repair requires complex, overlapping, interdependent processes with multiple vascular, cellular, and biochemical responses. The actual time frame for healing varies depending on several factors, such as type of wound closure (e.g., primary or secondary intention) and patient factors (e.g., perfusion, nutritional status, edema, tissue oxygen tension, presence or absence of infection, and comorbid metabolic diseases). Generally, in acute wounds, hemostasis and the inflammatory phase begin the first day after injury. Migration and the proliferation phase begin shortly after inflammation and peak at 7 to 21 days. Remodeling and wound contraction begin about 3 weeks after injury and may continue for 6 months to a year (Figure 16-3).8 The first days and weeks after injury are critical periods in the healing process because multiple cellular events occur.
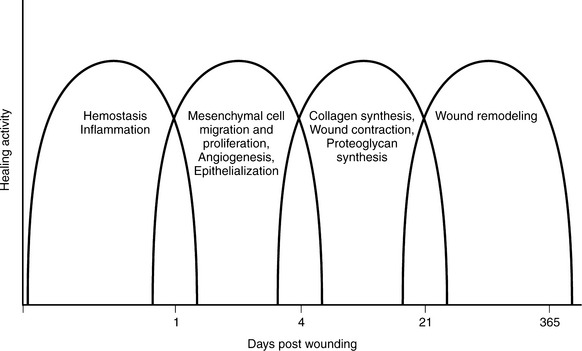
FIGURE 16-3 Phases of the healing wound.
(From Lawrence WT: Physiology of the acute wound, Clin Plast Surg 25:321-340, 1998.)
HEMOSTASIS AND INFLAMMATION
When a wound extends through the epidermis, blood vessels are disrupted and collagen is exposed, activating the clotting cascade and initiating an inflammatory response. A clot is formed at the injured site and vascular vasospasm occurs, providing hemostasis. The clot that forms is made of platelets, collagen, thrombin, and fibronectin, and these factors release chemoattractants (e.g., cytokines) and growth factors that initiate an inflammatory response. The fibrin clot, in addition to hemostasis, provides a scaffold for the migration and proliferation of cells.2–5,9–11 The initial response to injury and formation of a clot attract inflammatory cells (i.e., neutrophils) and several chemoattractants and growth factors, which orchestrate the inflammatory response.9,10
The complement system is activated with inflammation. Components of the complement system are proteins that facilitate bacterial destruction. Vasoconstriction at the wound site curtails blood flow and depletes oxygen delivery to the wounded tissues. Hypoxia after immediate wounding stimulates macrophages and initiates angiogenesis. Angiogenesis is the development of new blood vessels, a process that is essential in the proliferative phase of wound healing. Macrophages are considered vital to wound healing because they secrete essential cytokines that debride the wound, stimulate fibroblasts to produce collagen, promote angiogenesis, and stimulate keratinocytes.2–5,11 Prolonged hypoxia impairs wound healing because it impairs the function of neutrophils and macrophages, increasing the risk of bacterial invasion.4 Vasodilation of adjacent vessels follows vasoconstriction at the wound site, increasing perfusion and permitting plasma proteins to migrate into the wounded area with consequent edema, erythema, and pain.4,11 Increased metabolic activity and blood flow elevates the temperature of the wound and surrounding tissue, resulting in the symptoms of erythema and warmth. Finally, fibroblasts respond to the multiple chemoattractants activated by the inflammatory process with stimulation of granulation tissue formation.
Inflammation is the body’s immune system reaction to injury and is essential to normal wound healing.4 The early physiologic responses to tissue injury accomplish hemostasis through clot formation with initiation of an inflammatory response. This vital inflammatory response begins to clear the wound of cellular debris and activates a series of cellular interactions necessary for repair of the wound.
CELL MIGRATION AND PROLIFERATION
The processes of cell migration and proliferation predominate 2 to 4 days after wounding and are mediated by chemoattractants and growth factors. This phase of healing is characterized by epithelialization, angiogenesis, and formation of collagen. The primary migratory cells are epithelial cells (keratinocytes), fibroblasts, and endothelial cells. Goals of this phase are effective resurfacing of the wound.2–5
The migration of epithelial cells across a wound provides protection against entry of bacteria into the wound and fluid loss. Within 24 to 48 hours of wounding, epithelial marginal basal cells enlarge, flatten, undergo mitosis, and migrate to cover the wound bed. The epidermal covering, composed of primarily keratinocytes, begins to differentiate and re-establish the protective barrier. This process is referred to as epithelialization. Cells migrate from one wound edge to an adjacent wound edge and from dermal appendages such as hair follicles. Cells move across the denuded area in a leapfrog fashion.4,12 A moist wound environment enables the migrating cells to move across the wound surface more easily and quickly. Desiccation of wounds and eschar on the wound surface act as a deterrent to movement of epithelial cells. In wounds allowed to heal in a moist, protected environment, epithelial cells migrate on top of the wound. This is in contrast to wounds that epithelialize by cell migration under eschar that forms when a wound is allowed to become dry.
Angiogenesis occurs along with epithelialization, providing neovascularization of the wound bed. New tissue generation is dependent on nutrients supplied by the newly developed capillaries.2 Concurrently, fibroblasts migrate to the wound site to synthesize and deposit collagen and extracellular matrix. Fibroblasts are sensitive to the partial pressure of oxygen and acidosis.13 Local acidosis and hypoxemia initially stimulate fibroblast activity and angiogenesis; however, prolonged states of acidosis and hypoxemia will inhibit these same cells and their functions, slowing wound healing. Fibroblasts proliferate and synthesize collagen and extracellular matrix, resulting in granulation tissue covering the wound bed. New granulation tissue first appears as pale pink buds; as it fills with new blood vessels, it becomes bright, “beefy,” and red.4 This newly formed granulation tissue is very fragile.
New collagen provides strength and support to new tissues through its deposition and cross-linking in the injured area. Collagen is both synthesized and degraded in a continual process of wound repair. Over several weeks the collagen is remodeled and this process of collagen reorganization results in a stronger, tighter wound matrix. Unfortunately, the new tissue lacks the tensile strength of uninjured tissue and its strength is estimated to be no more than 80% that of healthy uninjured tissue.5
Wound contraction occurs during the proliferative phase of wound healing. It is the active process by which the area of a wound is decreased by contracting the extracellular matrix and wound edges. In this process new tissue is not formed; inward movement of existing tissue at the wound edge closes the area of the wound. Myofibroblasts, in granulation tissue, extend and retract pseudopods attached to collagen fibers, contracting the wound bed slowly (0.6-0.75 mm/day).2–5,9–13 Wound contraction and remodeling will occur for approximately 6 to 24 months after injury.
REMODELING
As healing progresses, edema decreases and the numbers of fibroblasts recede. The local metabolic needs of the tissue decrease and the tissue enters into the final repair process, remodeling. This begins approximately 3 weeks after injury and continues for up to 24 months. The collagen is reorganized to increase strength of the new tissue. If there is excessive collagen synthesis, then a hypertrophic scar or keloid can result.2–5 As the scar tissue matures, it also generally changes color and form. The early red, edematous, firm scar softens, lightens to pink, and becomes smaller. The scar tissue is strengthened through remodeling; however, the tissues only achieve approximately 80% of their original strength.
TYPES OF TRAUMATIC WOUNDS AND SOFT TISSUE INJURIES
CONTUSION
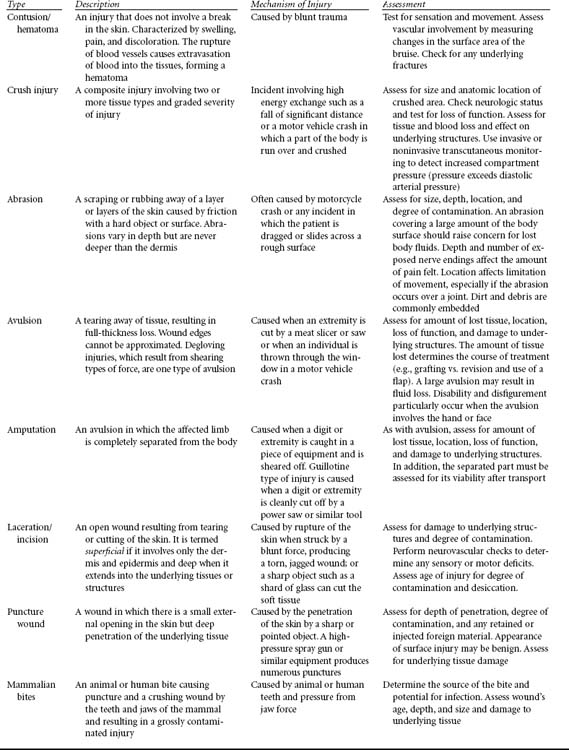
A contusion, or bruise, arises from rupture of subcutaneous blood vessels and extravasation of erythrocytes (Table 16-2). There is no break in the skin, but discoloration or ecchymosis, swelling, and pain are present at the site of contusion. A hematoma may accumulate at the site of ecchymosis.
HEMATOMA
A hematoma, resulting from rupture of a deeper and larger vein or artery, will expand in soft tissues until pressure in the area of hemorrhage exceeds the pressure within the disrupted artery or vein (see Table 16-2). Thus arterial hematomas, elaborated by arteries under greater pressure (mean arterial pressure is approximately 80-100 mm Hg) accumulate at a more rapid rate and to a larger size than do hematomas of venous origin. The size of the hematoma is also related to the capacitance of the tissues in which it forms. For instance, in the hand, where the tissues are not so distensible, the pressure will rise quickly as the skin starts to distend and tamponade the extravasation. However, in a region such as the thigh, several liters of blood may extravasate because the soft tissue envelope is more accommodating.
ABRASION
Abrasions suggest a friction mechanism of injury such as dragging (see Table 16-2). These injuries may be superficial (i.e., involving only the epidermis or partial thickness of the dermis) or they may be deep (i.e., violating the full thickness of the dermis). A partial-thickness abrasion demonstrates erythema and punctate bleeding and is painful. It is not unlike a partial-thickness burn. A full-thickness abrasion is white, does not bleed, and is painless because of injury to the sensory nerves as well. A partial-thickness abrasion may progress to a full-thickness loss of skin if it is not treated appropriately or if systemic resuscitation is suboptimal, resulting in tissue hypoxia and ischemia.
Traumatic abrasions are often contaminated with debris implanted into the skin, resulting in traumatic tattooing.14 Gravel or road debris may become embedded, for instance, in a patient ejected during a motor vehicle crash. This mechanism of injury is suggested by linear streaking within the abrasion.
AVULSION
Avulsions result from stretching or tearing away of the soft tissues, creating a full-thickness loss (see Table 16-2). Unfortunately, the magnitude of an avulsion injury is often underestimated. Tissues that appear viable and salvageable on admission are frequently devitalized and unsalvageable the following day. This scenario is often repeated every 24 hours, with progressive loss of tissue. The tissue that remains behind is thus significantly compromised and declares itself viable or not over 48 to 96 hours.
LACERATION
Lacerations, compared with other soft tissue injuries, can appear to be a more elementary problem (see Table 16-2). Lacerations may be caused by trauma with a sharp object such as glass or a knife wound. The adjacent area of crush and devitalization in these mechanisms may be small, approaching surgical incisions. These linear lacerations approximate well and have an optimal chance to heal. Lacerations from a blunt mechanism of injury are associated with larger margins of contused and compromised tissues. Often visible around the laceration is a halo of erythema or ecchymosis, indicating that the absolute zone of injury is larger than that of a laceration caused by the sharp object (e.g., a knife), with a relative zone of injury quite large.
PUNCTURE WOUND
Puncture wounds carry a heightened risk of infection (see Table 16-2). Although they do not cause vast soft tissue destruction or lacerations, puncture wounds can set up an aggressive infection because they deliver bacteria or foreign inoculum deep into the body. Puncture wounds should not be closed so that any infections that may develop can be optimally treated. Animal bites are notorious causes of puncture wounds. A bite from a dog with large teeth often causes lacerations that have a lower likelihood of getting infected because the bacteria can work their way out. However, in contrast, because of their fine, needlelike teeth, cats can cause a deeper bacteria inoculum that seals over. Bacteria then flourishes and cannot egress, leading to a virulent infection.15
DETERMINANTS OF THE HEALING PROCESS
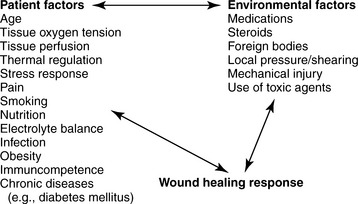
Physiologically, wound and soft tissue healing begins at the moment of injury and proceeds until tissue continuity is re-established. Several local and systemic factors influence the healing process. These can be organized conceptually into a human response model that includes factors inherent in the person that increase vulnerability for impaired healing and environmental factors that present a risk for impairment of healing (Figure 16-4). Some factors may be modifiable, whereas others cannot; all are worthy of consideration to provide appropriate therapy and an environment that supports healing.
AGE
Advancing age influences healing. The elderly have slower cellular activity and multiple concurrent conditions, predisposing them to impaired healing.16 In aging skin the epidermis thins gradually and is more easily stretched because of a decrease in elastin fibers. The dermal layer undergoes many changes with aging. There is a loss of approximately 20% in dermal thickness, which may account for the thinning appearance of elderly skin. Decreases in dermal cells, blood vessels, nerve endings, and collagen alter thermoregulation, sensation, and protective functions (e.g., moisture retention) of the skin with aging. The subcutaneous layer atrophies, decreasing the mechanical protection and insulation provided to the dermis. Additional factors such as lowered immunologic resistance, circulatory changes, and poor nutritional status seen in the elderly also contribute to altered wound healing. Older individuals frequently have more comorbidities (e.g., coronary artery disease, diabetes, pulmonary disease, etc.) that also compromise wound healing.5 Because of these changes, aging skin is more prone to traumatic injury and generally takes longer to heal.
TISSUE OXYGEN TENSION
Oxygen is essential to meet the energy needs of biologic activity. In wounds, oxygen and perfusion play critical roles in the healing process and are related to many patient factors that influence healing. Ischemic and hypoxic tissues do not heal. An adequate supply of oxygen to the wounded area is needed for phagocytic activity of neutrophils and macrophages, angiogenesis, epithelialization, and synthesis and accumulation of collagen,2–5,9–13 Compromised oxygen delivery to the wounded tissue will result in higher risk of infection, development of weaker collagen, and decreased tensile strength in the repaired tissues.2,5,10,17 The rate of epithelialization is also dependent on tissue oxygen tension. Keratinocyte replication and migration requires oxygen.
Initially the disruption of the blood vasculature creates a local hypoxemic state that stimulates cytokines and growth factors to begin angiogenesis. Once angiogenesis begins, the success of new endothelial development is dependent on an adequate tissue oxygen tension.2–5,9–13,17,18 Tissue oxygenation is essential for normal wound healing. It has increased importance when subcutaneous tissue and fascia are involved in the wounding process. Wound healing is slower in these tissues because of limited vascular supply and is further compromised in the presence of low oxygen tension.
PERFUSION
Oxygen delivery, along with the supply of neutrophils, macrophages, cytokines, growth factors, and nutrients, is closely linked to perfusion. Measurement of tissue oxygen tension provides information about blood flow to peripheral tissues. Decreases in tissue oxygen reflect insufficient blood flow, provided pulmonary status is normal. Changes in blood volume and perfusion during states of physiologic stress divert perfusion away from the skin, compromising oxygen delivery to cutaneous tissues.17,18 Thus, tissue perfusion assessment should include evaluation of local vasoconstriction compromise at the wound as well as general perfusion (e.g., shock states).5
Multiple factors influence perfusion in the trauma patient. Excessive catecholamine release with traumatic injury and hypothermia induce vasoconstriction, which will compromise tissue oxygen tension. Intraoperative and vasoactive agents used to support the cardiovascular system may compromise peripheral perfusion to tissues.2,5,10,17,18 Sepsis and systemic inflammatory response syndrome may further alter tissue perfusion and oxygenation through maldistribution of blood flow and excessive cytokine release.9,17 Interventions to maximize perfusion to enhance wound healing should be addressed in the trauma patient’s plan of care. Management should include support of cardiovascular and pulmonary systems, sometimes above normal, to preserve wound perfusion and tissue oxygenation.
SEVERE ANEMIA
Several factors can restrict tissue oxygen supply. Severe anemia is a patient element that influences tissue oxygen and, potentially, healing. Because oxygen is transported primarily by hemoglobin, there is concern that oxygen supply will be limited in patients with severe anemia. However, a number of studies indicate that anemia is not as serious a threat to healing as was once thought. Anemia in the presence of normal vascular volume and cardiac function does not impair wound healing until the hematocrit reaches a very low level (15%-18%).13 At that point, transfusion is beneficial in terms of maintaining tissue oxygen supply.
INFECTION
Again, tissue oxygenation is essential for the prevention and treatment of wound infection. Oxygen is used in the aerobic pathway of leukocytes for the killing of bacteria that have been introduced or that have migrated to the wound site. Oxygen is converted into free radicals that form a system of bactericidal agents. As leukocytes phagocytose bacteria, a primary oxidase in the cell membrane is activated that catalyzes an oxidation reaction, with subsequent killing of the bacteria. Recent evidence has shown that sufficient tissue oxygen levels are needed to resist infection.10,17
Immuncompetence of the patient will also influence the ability to resist wound infection. States of chronic stress, sepsis, and disease or drugs that compromise immunologic cells will deter the healing process but not absolutely inhibit healing.2–5 A patient with a compromised immune system may require more meticulous wound cleansing and vigilance in the assessment for wound infection and progression of healing. The ability of the patient to mount an inflammatory response is essential to normal healing. Equally important, is that the inflammatory process proceed in an orderly fashion (i.e., initiate and cease) for wounds to heal. Wounds in which the inflammatory process is prolonged because of local or systemic factors heal more slowly and may be categorized as chronic.13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree