CHAPTER 82 Vertebral Artery Injuries Associated with Cervical Spine Trauma
Epidemiology
As improved, higher-resolution imaging protocols have been developed and instituted, the incidence of detectable VAIs has increased. Although VAI has been reported as a result of low-energy movements such as chiropractic manipulation,1,2 yoga, and sudden head turning,3–5 discussion of incidence is most appropriate for consideration in high-energy cervical spine trauma such as motor vehicle collisions and falls.
Investigation into incidence of VAI began as reports on small cohort groups and then later matured into larger, prospective studies. Louw and colleagues6 were the first to investigate VAI in bilateral facet dislocation in a prospective fashion using digital subtraction angiography (DSA) and detected arterial occlusion in 9 of 12 (75%) patients. Willis and colleagues7 defined “high-risk” groups as those with criteria of either cervical spine fracture involving the transverse foramen or bilateral facet dislocations and, using DSA, detected VAI in 12 of 26 (46%) patients. Miller and colleagues8 described a broader definition of “high-risk” criteria including (1) cervical spine fracture/dislocation, (2) neurologic findings not explained by brain imaging, (3) Horner syndrome, (4) Le Fort II or III fracture, (5) skull base fracture, or (6) expanding neck soft tissue injury. Of 216 patients who met the criteria, they found 49 (22.7%) VAIs. Biffl and colleagues’9 selection criteria was even more inclusive and found 92 VAI among 605 patients (15.2%). Using magnetic resonance angiography (MRA), Vaccaro and colleagues10 noted a 37% rate of VAI with unilateral and bilateral cervical facet dislocations. In a follow-up study Vaccaro found a 20% (12/61) incidence of VA occlusion in patients with subaxial cervical spine fractures.11
Later epidemiologic studies have used large, level I trauma registries to more accurately define overall incidence of VAI in blunt trauma admissions. The incidence of VAI among total blunt trauma admissions ranged from 0.075% to 0.77%.8,12–13 The studies had inherent variations due to location and referral pattern of the center and different imaging modalities used including digital subtraction angiography, computed tomography angiogram, and magnetic resonance arteriography. Despite the relatively low overall incidence of blunt VAI in the overall trauma population, this small population of patients has a substantial risk of stroke and mortality,14 thereby demanding screening criteria and structured protocols for detection of clinically silent lesions.
Mechanism and Types of Arterial Injury
Although VAI has been described at all levels, the V2 segment is the most commonly cited level of injury due to its confinement and tethering within the foramen transversarium, making it susceptible to shear forces during forceful cervical rotation or direct injury from fracture fragments.15,16 Although less common, the tortuous V3 segment is vulnerable to injury due to its tethering between the foramen transversarium of the atlas and the atlantal-occipital membrane.17
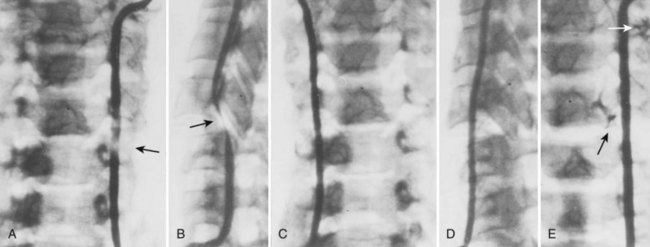
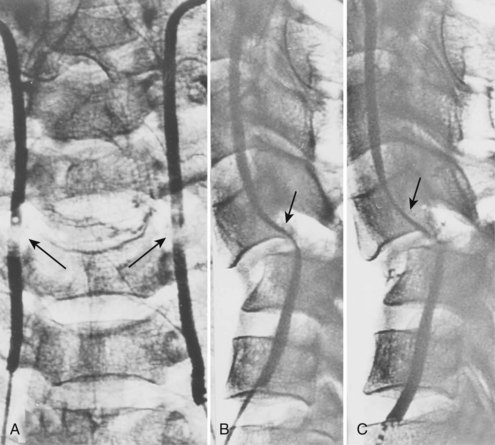
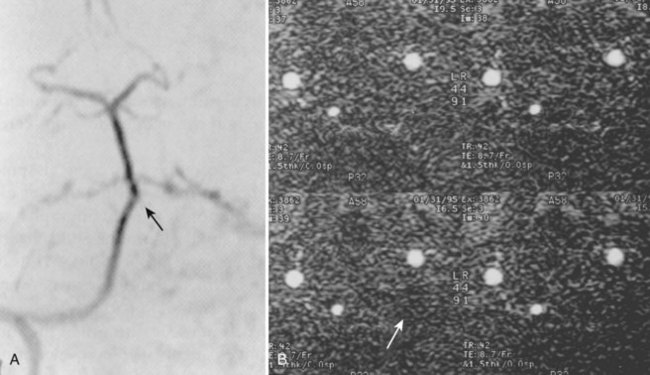
Cervical flexion-distraction fracture patterns most commonly result in VAIs, followed by flexion-compression injuries (Figs. 82-1 and 82-2).11 A review of the literature suggests that a rotational component to the cervical spine may also be a risk factor for VA occlusion.
In an attempt to better refine patient screening criteria for VAI, Cothren and colleagues13 reviewed 17,007 blunt trauma patients of whom 125 had cerebral vascular injuries associated with cervical spine injuries. The injuries were characterized as subluxations in 56 (48%) patients, C1 to C3 cervical spine fractures in 42 (36%), and extension of the fracture through the foramen transversarium in 19 (16%). Cervical spine fractures were the sole indication for screening in 90% of the study population. Screening yield of all patients admitted with one of these three fracture patterns was 37%. Therefore they recommended focused screening for patients with cervical subluxations, fractures through the transverse foramen, or fractures of C1 through C3.
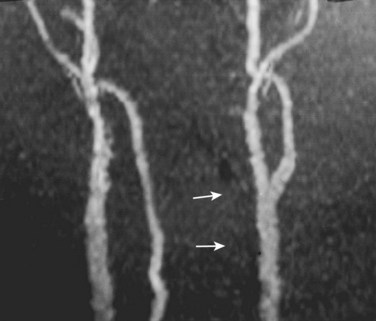
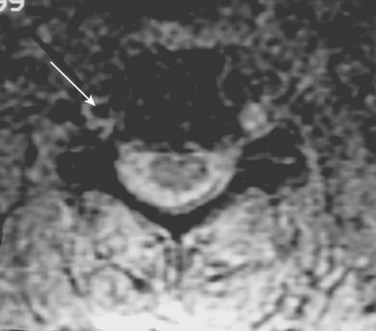
Injury to the VA in the setting of nonpenetrating cervical trauma is most likely due to a stretching mechanism.18 Sim and colleagues19 demonstrated in a cadaveric cervical spine model that the static deformity of a flexion-distraction stage II to IV subaxial cervical injury resulted in significant compression/stretch of the vertebral arterial vasculature (Figs. 82-3 to 82-5). Initially the vessel, or a portion of its intimal lining, is injured through excessive distraction in a flexion-distraction injury owing to the soft tissue attachments of the vessel in the foramina transversaria. Secondary events such as thrombus formation may then lead to occlusion of the vessel lumen. Recanalization over time after blunt injury to the VA was not a feature observed in a long-term follow-up study reported by Vaccaro and colleagues10 of image-confirmed vertebral vessel disruption. The lack of recanalization suggests that significant vessel intimal disruption occurs and that thrombus formation is not the only factor involved in vessel occlusion.
The intima of the VA is highly sensitive to shear forces experienced during high-energy blunt trauma. Because the vessel adventitia is more resilient, isolated intimal disruption can lead to a spectrum of clinical entities including an intimal flap with resultant thrombus formation or a dissection as the intima and adventitia separates cranially. Thrombus and dissection can lead to occlusion. If the energy transmitted is high enough, the adventitia can fail, leading to a pseudoaneurysm formation20 or transection if complete.
DSA provides the most anatomic detail of arterial injuries. A dissection of the VA has been shown to be the most common pattern of VAI, followed by occlusion. Multiple studies have demonstrated that dissections tend to resolve, whereas occlusions, as a rule, do not recanalize regardless of therapeutic attempts.10,21,22
Clinical Diagnoses
Abundant collateral circulation feeding the vertebrobasilar system and posterior circulation of the brain often allows unilateral disruption of the VA to remain asymptomatic. When this collateral circulation is diminished in situations of atherosclerosis or anatomic variations, patients may initially present with symptoms of vertebrobasilar insufficiency. Symptoms of VA insufficiency may manifest as dizziness, dysarthria, dysphagia, diplopia, blurred vision, and tinnitus.17
Initially asymptomatic patients can have progression of symptoms either acutely or delayed due to embolism, thrombus extension, or dissection. Heros and colleagues23 described a patient who sustained a VAI with a resultant cerebellar infarction despite having a normal contralateral artery. To explain the infarction, the authors postulated that the thrombosis had extended distally to the intracranial portion of the VA. Interestingly, Six and colleagues24 reported a case of an asymptomatic post-traumatic bilateral VA occlusion. Angiography demonstrated occlusion of both vertebral arteries but with the presence of blood flow through the intramuscular collateral vessels of the thyrocervical trunk and by collaterals from the superficial occipital artery. In fact, Blam and colleagues25 conducted a retrospective review of 1283 patients with cervical spine trauma and determined that a normal neurologic examination is not predictive of VA patency after significant cervical spine trauma. These authors also noted that VAI was observed in similar frequency in both neurologically intact and motor incomplete patients (ASIA C and D).25
The interval between spinal injury and the development of vertebrobasilar symptoms varies greatly from immediately after trauma to up to 3 months later.17,26 The late onset of symptoms suggests a process of vertebral vessel thrombus formation at the injury site with clot propagation or embolism and subsequent infarction. Therefore clinicians should be aware that late symptoms and signs of vertebrobasilar insufficiency after cervical spine trauma may be a manifestation of VAI that occurred at the time of trauma.11 Vaccaro and colleagues10 demonstrated that 83% of patients with a known traumatic vertebral vessel injury demonstrated no flow reconstitution after an average follow-up of 25.8 months. The one patient who did show reconstitution of flow was thought to have a resolution of vertebral arterial spasm that initially compromised flow.