Vascularized Grafting for Femoral Head Osteonecrosis
Sameer Lodha
Robert W. Wysocki
Case Introduction
A 39-year-old female presented to clinic for evaluation of left hip pain. She stated that she had had mild, intermittent hip pain for approximately 1.5 years previously, but had experienced a significant worsening over the past month. She described the pain as a deep ache localized primarily to her left groin, and worse with weight bearing and with internal rotation. At its worst, she rated the pain an 8 to 9/10. She denied any significant exposure to corticosteroid therapy and also denied any history of significant alcohol consumption. There was no history of trauma to the left hip. She denied any history of HIV or other immune disorders. There was no family history of hip disorders, hemoglobinopathies, or coagulopathies.
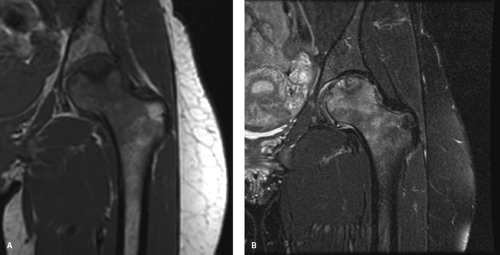
Physical examination revealed an antalgic gait, favoring her left lower extremity. On ROM testing, internal rotation was painful and limited by 30 degrees as compared to the contralateral hip; there was also a 10-degree loss extension, and 20-degree loss of abduction. Plain radiographs revealed sclerotic and cystic changes in the femoral head, with an area of collapse in the medial aspect of the weight-bearing portion of the head. This collapse was noted to be less than 1 mm (Fig. 58.1). An MRI scan was obtained which supported a diagnosis of osteonecrosis, with head involvement of approximately 30% (Fig. 58.2).
Indications and Contraindications
Osteonecrosis (or avascular necrosis) of the hip is a relatively common, potentially disabling disorder involving impaired osseous blood flow to the femoral head. Approximately 300,000 to 600,000 people are estimated to be affected in the United States alone, with 10,000 to 30,000 new cases
identified each year (1,2). Patients are usually male, and present at a relatively young age, typically in the third to fifth decade of life (3). Inadequate treatment and disease progression can result in the development of significant arthrosis of the femoroacetabular articulation, potentially requiring hip arthroplasty or arthrodesis as a salvage procedure. Indeed, osteonecrosis is estimated to account for approximately 10% of total hip arthroplasties (4). Notwithstanding advances in arthroplasty technology, these continue to be less than ideal treatment options, given the characteristically relatively young age and high functional demands of this population. This fact has spurred considerable research into alternative treatment options. The etiology and natural history of hip osteonecrosis remain incompletely defined; however, and this has contributed to continued controversy over the optimal treatment for this challenging disease.
identified each year (1,2). Patients are usually male, and present at a relatively young age, typically in the third to fifth decade of life (3). Inadequate treatment and disease progression can result in the development of significant arthrosis of the femoroacetabular articulation, potentially requiring hip arthroplasty or arthrodesis as a salvage procedure. Indeed, osteonecrosis is estimated to account for approximately 10% of total hip arthroplasties (4). Notwithstanding advances in arthroplasty technology, these continue to be less than ideal treatment options, given the characteristically relatively young age and high functional demands of this population. This fact has spurred considerable research into alternative treatment options. The etiology and natural history of hip osteonecrosis remain incompletely defined; however, and this has contributed to continued controversy over the optimal treatment for this challenging disease.
Vascularized bone grafting offers two primary theoretical benefits over other treatments for osteonecrosis, including nonvascularized bone grafting: (i) improved incorporation of a “living” graft, and (ii) the introduction of a new blood supply to the femoral head via the graft itself. Although there are numerous options for the graft harvest site, the free vascularized fibular graft has been the best described and offers a number of advantages, including the relative ease of harvest of an expendable bone. Other valuable attributes of this graft are the ability to vary the length of the graft easily, and the reliable vascular supply. The location of the fibula permits two operative teams to work simultaneously on both harvesting the graft and preparing the hip, thus reducing overall operative time (5,6).
The specific factors that define an appropriate candidate for vascularized grafting remain somewhat controversial, especially in light of the recent advances made in hip arthroplasty (7). Urbaniak et al. (6), in their study of 103 hips with a minimum follow-up of 5 years, noted a conversion rate to total hip arthroplasty of 11% in precollapse hips—indicating that the best results for vascularized fibular grafting are obtained in hips in early, precollapse stages of disease. This compares favorably with the 65% conversion rate reported by Mont and Hungerford (2) in their study of nonoperatively treated precollapse hips.
Relatively successful results have also been obtained in postcollapse hips. Berend et al. evaluated 224 postcollapse hips treated with vascularized fibular grafting, with an average follow-up of 4.3 years. In hips with a minimum of 5-year follow-up, they noted a 65% survival rate. Larger lesion size, and certain etiologies (notably, idiopathic and alcohol abuse) were associated with worse outcomes (8). Further patient differentiation has showed that in postcollapse hips without depression, the conversion rate was 23%; for hips with collapse and depression, the rate was 39% (6).
The ideal candidate for vascularized fibular grafting is, therefore, a young patient, with early, precollapse disease. The procedure is still an option, however, for the younger patient with more advanced (stage 3 to 5) disease, and represents a viable avenue for avoiding or delaying conversion to a total hip arthroplasty. For older patients with predegenerative disease, the procedure is an option; however, the presence of a large lesion (>50% of the femoral head) with collapse we consider an indication for arthroplasty (3,9).
Surgical Technique: Vascularized Fibula Grafting
Preoperative preparation is important as the surgery involves many logistical and technical aspects including procurement
of proper equipment and coordination of the surgical teams. The surgery can be broken down into three essential parts: decompression and debridement of the femoral head, harvest of the fibular graft, and insertion of the fibular graft with microvascular anastomosis. If a surgeon is skilled and experienced in all these areas they can perform the entire operation with a single team. However, it is preferred that the surgery be performed with two surgical teams operating simultaneously and led by two separate surgeons proficient in the various elements of the surgery to decrease overall operative time.
of proper equipment and coordination of the surgical teams. The surgery can be broken down into three essential parts: decompression and debridement of the femoral head, harvest of the fibular graft, and insertion of the fibular graft with microvascular anastomosis. If a surgeon is skilled and experienced in all these areas they can perform the entire operation with a single team. However, it is preferred that the surgery be performed with two surgical teams operating simultaneously and led by two separate surgeons proficient in the various elements of the surgery to decrease overall operative time.
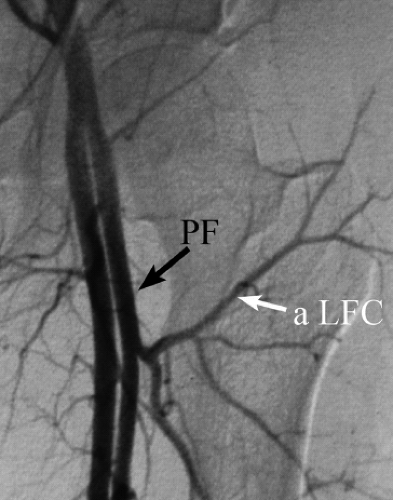
Preoperative arteriograms defining the vascular tree distal to the trifurcation of the femoral vessel are not routinely necessary. In an initial series of 400 patients, Urbaniak et al. found an absent anterior tibial, posterior tibial, or peroneal artery in only two (0.005%) (3). Arteriograms are now only recommended in cases where there has been previous trauma or surgery, or where either the dorsalis pedis or posterior tibial vessels are not palpable or obvious to Doppler examination (Fig. 58.3).
The complex nature of the case requires equipment of various types. It is helpful to make a list (Table 58.1). The surgical time allotted (3 to 6 hours) will depend on the experience of the surgical team and technical difficulty of each particular case. Anesthesia should be general endotracheal supplemented with an epidural catheter for postoperative pain control. Blood products are not usually necessary as blood loss is typically minimal. A Foley catheter should be placed to monitor urine output and for later comfort. An infusion of Dextran 40 at 20 cc/hr is started at the beginning of the case to provide a measure of anticoagulation.
Table 58.1 Surgery Equipment List | |||||||
|---|---|---|---|---|---|---|---|
|
The patient is placed on a beanbag in a lateral decubitus position with the affected leg up. All bony prominences and superficial nerves should be padded and an axillary roll placed under the chest. The C-arm and monitor should be positioned in front of the patient opposite the surgeon. The C-arm itself is brought over the patient in a “rainbow” position (Fig. 58.4). Before prepping and draping, the surgeon should test the C-arm to verify that AP and frog-leg lateral images can be easily obtained.
The leg is prepped and draped to the iliac crest with the foot and toes wrapped in Coban leaving the ankle free. A sterile tourniquet is applied to the thigh just proximal to the knee and inflated exsanguination to 350 mm Hg or whatever standard pressure the surgeon may be accustomed to. Two surgical teams can work simultaneously. One team performs the hip approach and dissection of the recipient vessels and the other team harvests the fibular graft (Fig. 58.5).
Fibular Graft Harvest
A lateral incision made in line with the lateral malleolus and fibular head, approximately 15 cm long, limited to within 10 cm of each end of the fibula (Fig. 58.6). Dissection is carried down to the deep fascia, which is incised over the fat stripe of the lateral compartment for the full length of the incision. The peroneal muscles are sharply reflected off the posterior intermuscular septum from lateral to medial until the fibula is encountered. As the dissection then proceeds over the
fibula toward the anterior intermuscular septum, 1 to 2 mm of muscle is preserved creating a “marbled appearance” over the fibula, so as not to disturb the periosteum and its blood supply for the graft. The anterior intermuscular septum is then incised and the anterior compartment musculature with the anterior tibial artery and deep peroneal nerve are bluntly dissected off of the underlying interosseous membrane.
fibula toward the anterior intermuscular septum, 1 to 2 mm of muscle is preserved creating a “marbled appearance” over the fibula, so as not to disturb the periosteum and its blood supply for the graft. The anterior intermuscular septum is then incised and the anterior compartment musculature with the anterior tibial artery and deep peroneal nerve are bluntly dissected off of the underlying interosseous membrane.
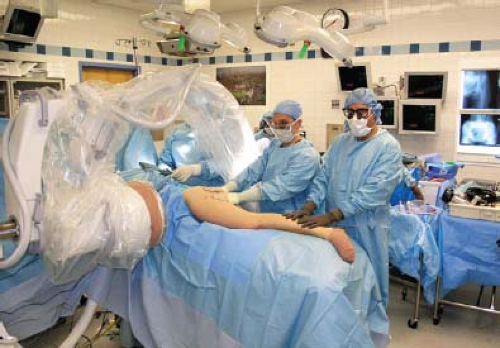 Figure 58.4. Patient in lateral decubitus position on beanbag. C-arm on chest side in “rainbow” position arching over patient. Both teams work simultaneously at hip and fibula. |
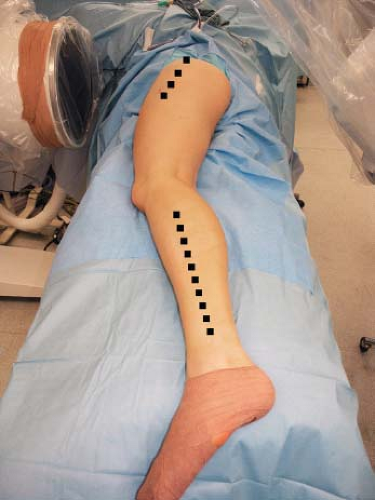 Figure 58.5. Operative leg draped from iliac crest including foot, demonstrating areas of hip and lower leg incision. |
After measuring a fibular harvest segment of approximately 15 cm for the average adult patient, retaining 10 cm proximal and distal to it, the osteotomies are now per-formed. A pair of mini-Hohmann retractors is placed around the fibula protecting the distal vascular pedicle. An oscillating saw is used to complete the osteotomy with copious saline irrigation to prevent thermal necrosis. The proximal osteotomy is completed in a similar manner.
 Figure 58.6. Lateral leg incision is made from distal and proximal 10-cm ends of the fibula. Central portion of fibula harvested based on pedicle. |
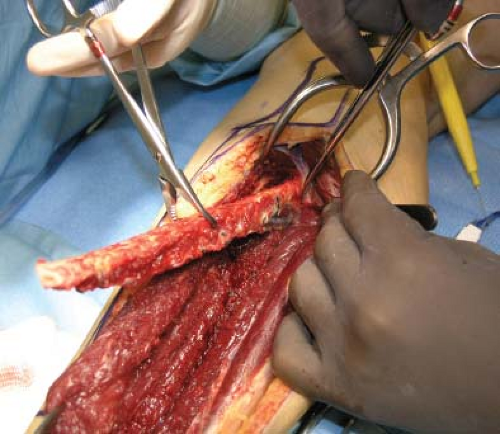 Figure 58.7. Fibula graft carefully lifted out of its bed. Note use of “lobster” claw clamp to rotate the fibula and control its removal. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree